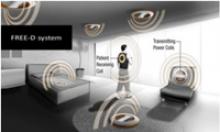
September 1, 2011 – Mechanical pumps to give failing hearts a boost were originally developed as temporary measures for patients awaiting a heart transplant. But as the technology has improved, these ventricular assist devices commonly operate in patients for years, including in former vice-president Dick Cheney, whose implant this month celebrates its one-year anniversary.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now