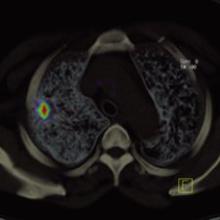
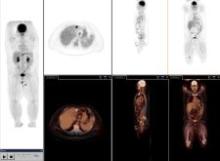
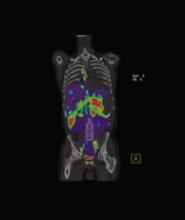
May 3, 2007 — According to a Dow Jones News Service article published by Star Tribune, Minneapolis St. Paul, Boston Scientific Corp. is reviewing its portfolio and will consider selling parts that aren't considered strategic, long-term contributors — so stated the company's chief operating officer Wednesday.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now