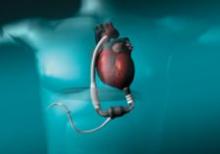
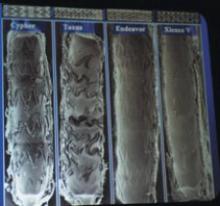
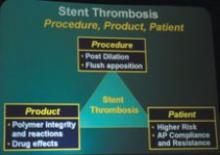
Barco has a new suite of clinical application modules available within its flagship 3-D software product, VOXAR 3-D. The CARDIAMETRIX suite provides radiologists and cardiologists with a powerful set of tools for structural and functional analysis of contrast-enhanced Computed Tomography Angiography (CTA) studies.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now