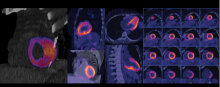
Nuclear imaging technology for both single photon emission computed tomography (SPECT) and positron emission tomography (PET) have made advancements in the past couple years. The main drivers for this have been a movement to digital imaging detectors to improve image quality and address radiation dose concerns, reimbursement and radiotracer supply issues.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now