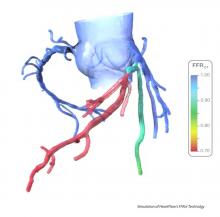
Aug. 1, 2017 — LimFlow SA announced publication of positive results from the pilot study of the LimFlow Percutaneous Deep Vein Arterialization System (pDVA) in the July issue of the Journal of Endovascular Therapy.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now