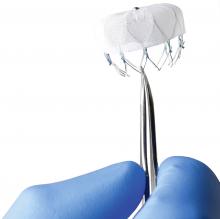
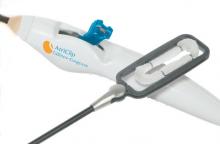
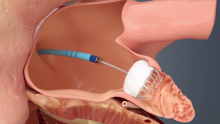
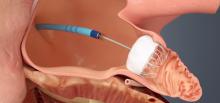
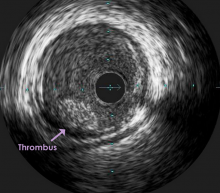
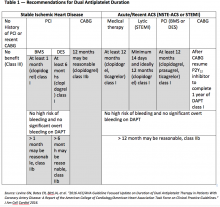
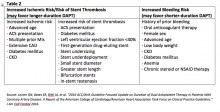
Using optimal frequency domain imaging (OFDI) to guide percutaneous coronary intervention (PCI) with second-generation drug eluting stents (DES) achieves equivalent clinical and angiographic outcomes to intravascular ultrasound (IVUS)-guided PCI at 12 months.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now