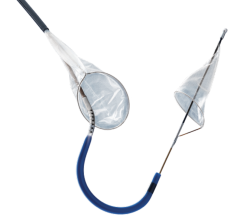
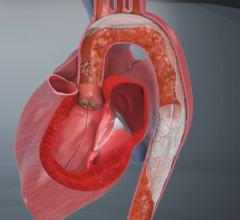
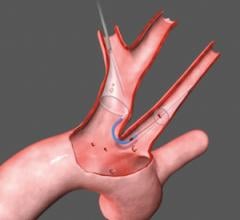
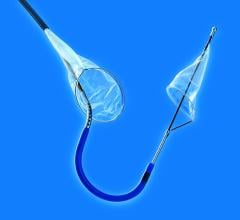
June 12, 2009 - Abbott today launch its sixth-generation Emboshield NAV6 Embolic Protection System for use in carotid artery stenting procedures.
Carotid artery stenting provides a minimally invasive treatment alternative to conventional open carotid artery surgery for patients who are at high risk for surgery. Embolic protection systems are used during the stenting procedure to prevent particles of dislodged plaque from flowing to the brain, potentially causing an ischemic stroke. The Emboshield NAV6 is now available in the U.S. and Europe.
The Emboshield NAV6 improves deliverability and ease of use for physicians, the company said. Abbott's proprietary BareWire technology allows for wire movement independent of the Emboshield NAV6 filter, giving physicians an increased level of control during carotid stenting procedures.
"The innovative design of the Emboshield NAV6 offers marked improvements to an already good embolic protection system. It provides excellent steerable wire options to independently reach challenging lesions, a short basket length, significantly improved visibility, an easier-to-use retrieval system, and optimal pore size and distribution," said D. Christopher Metzger, M.D., FACC, of the Holston Valley Medical Center, Kingsport, Tenn. "These improvements make the NAV6 system an outstanding embolic protection device."
Safety and efficacy endpoints for the Emboshield NAV6 were met in Abbott's PROTECT clinical trial, which was designed to examine carotid artery stenting with Abbott's Emboshield systems in patients at high-risk for carotid endarterectomy. In addition to demonstrating continued improvements in outcomes for carotid stent procedures, the PROTECT study data showed a low 1.8 percent composite rate of all stroke and death at 30 days in 220 patients. This rate is well within American Heart Association (AHA) 30-day all stroke and death rate guidelines for carotid endarterectomy of 6 percent for symptomatic and 3 percent for asymptomatic patients with carotid artery disease. The PROTECT study results were presented at the Transcatheter Cardiovascular Therapeutics conference in Washington, D.C., in October 2008.
"As a member of the PROTECT multispecialty Executive Committee, I have been impressed with the continuing downward trend in stroke rates with protected carotid stenting for patients at high risk for carotid endarterectomy from the earliest clinical trials with first-generation devices 10 years ago," said Jon S. Matsumura, M.D., professor of surgery, University of Wisconsin School of Medicine and Public Health. "These results are related to several factors, including process of care improvements that skilled physicians are developing, and to the continuing improvements in device technology. Abbott's PROTECT study represents state-of-the-art skills and technology for carotid artery stenting."
Abbott has developed a broad portfolio of technologies to help physicians provide better treatment for their patients with peripheral artery disease. The company is committed to making investments in endovascular therapies and educational programs and to designing and conducting endovascular clinical trials.
For more information: www.abbott.com


 April 25, 2023
April 25, 2023