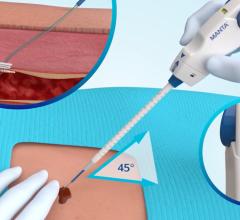
June 23, 2009 – AccessClosure yesterday launched its newest addition to the Mynx Vascular Closure family, the Mynx M5, which can be delivered directly through a small 5 Fr. cardiovascular sheath without enlarging the arterial hole or traumatizing the surrounding tissue in the process.
This “artery-friendly” approach also benefits hospitals, as the M5 eliminates the need for a sheath exchange, which saves procedure time and expense, the company said. Typically, procedural sheaths that are 5 Fr. in size are used in diagnostic coronary and peripheral catheterizations. The U.S. 5 Fr. closure market is estimated at nearly $200 million annually.
“Before the Mynx M5, existing closure devices required the physician to create a larger hole in the artery just to seal it in diagnostic cases,” explains Dharmesh Patel, M.D., FACC, a diagnostic cardiologist from Memphis Heart Clinic in Memphis, Tenn. “The M5 is the first device that specifically addresses the primary clinical need for the 5 Fr. market, which is to reliably close the puncture site without enlarging it first. This is particularly useful in my practice allowing patients to ambulate quicker with greater safety and tolerability.”
The Mynx Vascular Closure Device was first approved by the FDA for 6 Fr. and 7 Fr. cardiovascular procedures in May 2007. Since its full market release, the Mynx has been used in more than 225,000 patients in more than 700 hospitals nationwide.
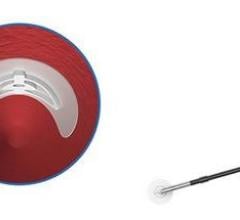
Designed with the patient in mind, the Mynx offers a new solution to help minimize the discomfort commonly associated with vascular closure due to its unique deployment method and novel sealant material, the company said. Previous closure devices have used metal or animal-based implants to seal the arterial hole left after a cardiovascular procedure. By contrast, the Mynx uses a soft, bio-absorbable polymer material. This sealant material, polyethylene glycol (PEG), has been used safely for more than a decade in a wide range of medical products such as gel caps and eye drops.
During the closure procedure, the sealant is placed gently over the arterial puncture area. The sponge-like sealant immediately expands three to four times its original size by rapidly absorbing blood around the puncture site, which immediately stops the bleeding and seals the artery. The sealant then dissolves naturally within 30 days, leaving nothing behind inside, or on top of, the artery.
For more information: www.accessclosure.com

 October 07, 2025
October 07, 2025