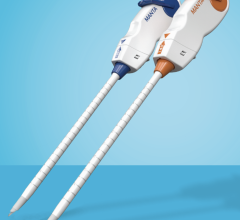
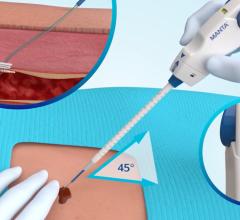
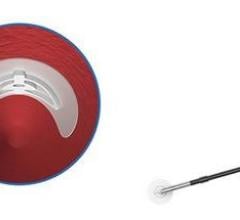
January 7, 2011 – A new access device is now available in cath labs across the United States. The Arstasis One Access Device, from Arstasis, is the first commercially available alternative to the Seldinger Technique in 52 years. Previously, only a select number of physicians participating in Arstasis clinical trials and select pre-launch registries have been able to use the device. "We are delighted to begin providing U.S. cardiologists with a femoral artery access device that allows them to perform angiography without resorting to inserting a vascular closure implant into the patient," said Bruce Modesitt, CEO of Arstasis. Along with the U.S. launch, patient enrollment is continuing in the RECITAL study. The non-randomized, prospective, post-market registry is anticipated to enroll up to 500 patients in at least seven U.S. hospitals. Since 1959, physicians have been using the Modified Seldinger Technique to insert flexible catheters into the femoral artery for performing procedures in the patient's arterial-vascular system. It is estimated that the most prevalent such procedure, diagnostic angiography, is performed more than half a million times per month worldwide. At the end of every such case, patients are left with a substantial hole in their femoral artery, which typically takes significant effort and cath lab resources to close. With the Arstasis One Access Device, however, physicians create a shallow-angle needle pathway through the wall of the femoral artery. When the sheath is withdrawn, the shallow-angle pathway collapses from the normal pressure of the patient's femoral artery blood flow and approximately three to four minutes of mild, non-occlusive finger-pressure. This quickly seals the access site. For more information: www.arstasis.com
If you enjoy this content, please share it with a colleague
Alternative to Arteriotomies and Vascular Closure Implants Now Available in U.S.
Related Content
Oct. 7, 2025 — Nobles Medical Technology II, a provider of cardiovascular closure solutions, has announced that the U.S ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
September 16, 2022 — Teleflex Incorporated, a leading global provider of medical technologies, announced that Dr. Magnus ...
November 9, 2021 — Results from the largest randomized trial available comparing different closure device strategies ...
July 15, 2021 — Vivasure Medical announced its development program for PerQseal Blue, a sutureless and fully ...
August 17, 2020 — Veryan Medical announced it will support Vasorum in the commercialization of the Celt atrial closure ...
Ashish Pershad, M.D., chief of interventional cardiology, Banner University Medical Center, Phoenix, explains the trend ...
April 3, 2019 — Essential Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for its large bore ...
December 20, 2018 — Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S. Food ...
November 21, 2018 – Vivasure Medical Ltd. recently announced the European launch of the PerQseal closure device for ...

 October 07, 2025
October 07, 2025