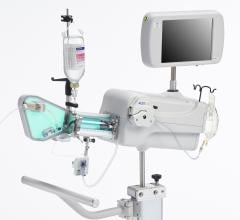
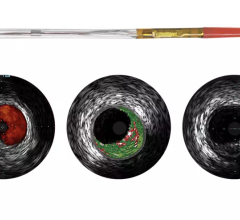
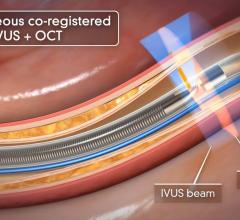
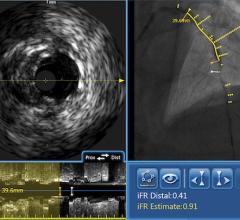
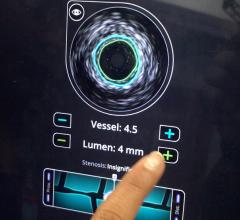
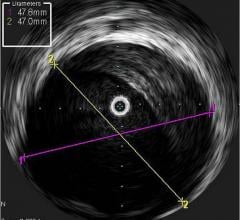
July 24, 2014 — Boston Scientific has initiated full commercial launch of its new Polaris imaging system. This system will support the Boston Scientific family of intravascular ultrasound (IVUS) catheters, including coronary, peripheral and intracardiac echo products. The Polaris system offers enhanced ease-of-use and more powerful processing capabilities. Its modular design also will support the planned release of new Boston Scientific imaging products including a fractional flow reserve (FFR) wire, a new family of IVUS catheters, enhanced software features and better system control tools.
Working in collaboration with physicians, nurses, technicians and software design experts, Boston Scientific identified a need to improve the experience for customers involved in intravascular imaging procedures. Common challenges included confusing workflows, increased procedure times and difficult image interpretation. The Polaris system is designed to be smart, fast and accurate. The user interface and workflow have been redesigned and enhanced to provide for greater ease of use, while providing the necessary information to guide clinical decisions.
The Polaris system has CE mark and U.S. Food and Drug Administration (FDA) 510(k) clearance. It has been evaluated in numerous hospitals in the United States and Europe, and was showcased during live cases at the EuroPCR conference in May.
"The Polaris system is an advance for intravascular imaging," said Lowell Satler, M.D., director of coronary interventions, MedStar Washington Hospital Center, Washington, D.C. "The improved ease-of-use and image processing simplifies the procedure and enables our team to quickly obtain the information needed to treat patients."
Boston Scientific will offer customers an opportunity to upgrade their existing iLab system to the new Polaris.
"There are overwhelming data demonstrating that using IVUS improves patient outcomes," said Isaac Zacharias, vice president and general manager, imaging, Boston Scientific. "The Polaris system represents the ongoing commitment of Boston Scientific to develop products that improve patient outcomes by providing physicians improved IVUS guidance in their daily practice."
For more information: www.bostonscientific.com


 September 18, 2025
September 18, 2025