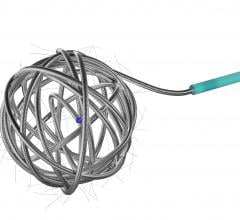
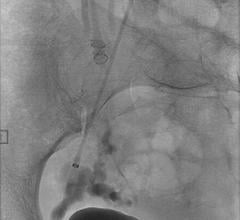
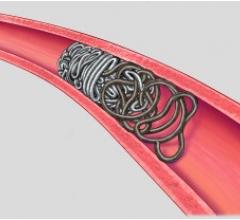
Micrus Endovascular Corp. launched the Cerecyte and stretch-resistant versions of its DeltaPaq microcoil system for the treatment of cerebral aneurysms.
The DeltaPaq system is designed to achieve 10 to 20 percent greater intra-aneurysmal packing density than conventional microcoils, the company said. Micrus develops, manufactures and markets implantable and disposable medical devices for use in the treatment of cerebral vascular diseases. The company’s three-dimensional microcoils automatically deploy within the aneurysm, forming a scaffold that conforms to a wide diversity of aneurysm shapes and sizes. Micrus also sells accessory devices and products used in conjunction with its microcoils.
Technology | October 30, 2008


 June 05, 2025
June 05, 2025