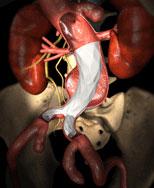
March 10, 2009 - Endologix Inc. today said the FDA approved the IntuiTrak Express Delivery System for the Powerlink XL stent graft.
The maker said the IntuiTrak Express enables delivery of the Powerlink XL stent graft through the IntuiTrak 19 Fr. introducer sheath during the endovascular repair of abdominal aortic aneurysms (AAA) in patients with aortic necks up to 32 mm in diameter.
Approval of the IntuiTrak Express provides additional treatment options to physician users and includes a 21 Fr. IntuiTrak stand-alone delivery system for the Powerlink XL stent grafts that incorporates its own integrated, hydrophilically coated introducer sheath with advanced hemostasis control.
The family of IntuiTrak Delivery Systems, first approved for U.S. commercialization in October 2008, represents an array of low-profile delivery systems with enhanced flexibility, advanced hemostasis control and hydrophilic coating to facilitate smooth delivery, particularly in patients with limited or difficult vascular access. The integrated introducer sheath eliminates the need for sheath exchanges in introducing ancillary devices during the endovascular AAA procedure, thereby offering the potential to reduce procedure time and blood loss, and minimize vessel trauma, the company said.
For more information: www.endologix.com


 April 26, 2023
April 26, 2023 









