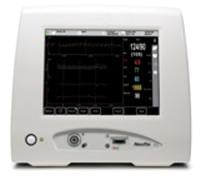
November 22, 2010 – The first noninvasive cardiovascular monitor with blood pressure and cardiac output measurements has been cleared by the U.S. Food and Drug Administration (FDA). The ccNexfin, by BMEYE B.V., combines the company’s noninvasive technology with Masimo rainbow SET Pulse CO-Oximetry for continuous hemoglobin and oxygen saturation monitoring.
The device provides real-time, beat-to-beat measurements of cardiac, circulatory and pulmonary parameters, which could help clinicians detect impending cardiovascular events. It utilizes a finger cuff to capture and continuously measure blood pressure, mean arterial pressure, cardiac output, stroke volume, systemic vascular resistance and derivative of pressure. The sensor also measures hemoglobin, oxygen saturation, oxygen content and perfusion index.
This detailed data allows clinicians to predict and proactively address the early signs of hemodynamic instability during critical situations rather than reacting to late indicators and their effects.
"ccNexfin has been very well received in the critical care market since its European launch in January this year. We are very excited to offer this unique totally noninvasive solution to U.S. physicians" stated Rob de Ree, CEO of BMEYE. "The combination of beat-to-beat blood pressure with cardiac output along with Masimo rainbow SET Pulse CO-Oximetry capabilities has the potential to provide earlier diagnosis and early goal-directed therapies in anesthesia and emergency care to improve patient care while reducing costs."
For more information: www.bmeye.com


 October 21, 2025
October 21, 2025 









