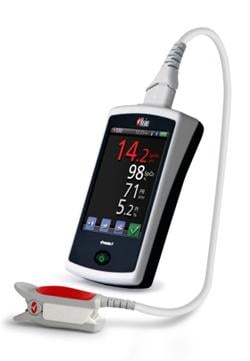
January 12, 2012 – Masimo announced U.S. Food and Drug Administration (FDA) 510(k) clearance and full market commercial launch of the Masimo Pronto-7—a palm-sized handheld device designed for quick and easy noninvasive spot-checking of total hemoglobin (SpHb), SpO2, pulse rate, and perfusion index.
Andrew J. Schuman, M.D.— adjunct assistant professor of pediatrics at Dartmouth Medical School, contributing editor for Contemporary Pediatrics, and a practicing pediatrician in New Hampshire, who reviewed the device prior to FDA clearance—said that the new Pronto-7 has the "potential for significantly improving pediatric practice."
"The Pronto-7 is a potentially game-changing device because it can help healthcare providers expedite the diagnosis and treatment of anemia in children and adolescents," said Schuman. “Not only does it represent an exciting medical advancement in point-of-care testing, but it also provides pediatricians with a high-tech alternative to painful venipuncture or finger-stick blood sampling that, until now, has been the only way to measure hemoglobin levels in our young patients. Parents and pediatricians will also appreciate our new ability to screen without the scream! Most would agree that the best preventative healthcare is provided both quietly as well as painlessly."
With FDA 510(k) clearance and full commercial availability of the new Pronto-7 device, which includes expanded sensor size options to accommodate a wider range of finger sizes and the addition of a Max Sensitivity Mode, clinicians throughout the United States will be able to quickly and conveniently measure total hemoglobin, SpO2, pulse rate, and perfusion index— without removing a drop of blood.
Hemoglobin is one of the most commonly ordered tests in both hospital and non-hospital settings because it is critical to assessing blood loss. However, current testing requires a painful needle stick for the patient, time-consuming blood draws for the clinician, and typically provides delayed results. The Pronto-7 offers a breakthrough solution for measuring hemoglobin in less than one minute—without the needles, risk of blood contamination, hazardous medical waste, laboratory analysis and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm and weight of 296 grams, the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, pulse rate, and perfusion index, into any clinician's hands in various clinical settings, including physician offices, hospitals and clinics. Because of the device's embedded 802.11 b/g and Bluetooth communication capability, wireless printing or emailing of test results is enabled and future upgrades will allow for wireless transmission to electronic health record (EHR) systems.
Thomas A. Murray, III, M.D., an obstetrician/gynecologist in Salem, Mass., said, "As one of the first users of the prototype Pronto-7, I was impressed with its ease of use right from the start. In a general ob/gyn office, anemia is a common disorder and the Pronto-7 allows me to accurately measure my patients' hemoglobin levels noninvasively, conveniently and quickly—right here on-the-spot in my office. My patients used to cringe at the idea of a finger stick and love that Pronto-7 is noninvasive. It is also much safer for my staff as there is no possibility of a needle stick injury or blood exposure. I have found the Pronto-7 to be very useful in my practice."
For more information: www.masimo.com


 October 21, 2025
October 21, 2025