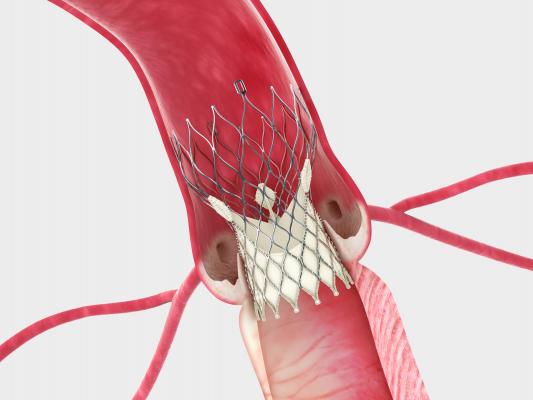
January 17, 2014 — The U.S. Food and Drug Administration (FDA) granted U.S. market approval for Medtronic’s self-expanding
transcatheter aortic valve replacement (TAVR) CoreValve System. It is the first self-expanding TAVR valve to be approved in the United States and the second TAVR valve to achieve FDA approval.
The FDA granted approval of the CoreValve device without an independent device advisory panel review after reviewing the clinical outcomes in the Extreme Risk Study of the CoreValve U.S. Pivotal Trial, which demonstrated that the CoreValve System is safe and effective with high rates of survival and some of the lowest rates of stroke and valve leakage reported.
The new valve is indicated for severe aortic stenosis patients who are too ill or frail to have their aortic valves replaced through traditional open-heart surgery. Untreated, these patients have a risk of dying approaching 50 percent at one year.
Court Challenge
While the CoreValve has achieved a major milestone to enter the U.S. market, it is tempered by a patent legal challenge by Edwards Lifesciences, maker of the Sapien valve, which up until today was the first and only FDA-cleared TAVR device in the United States.
On Jan. 15, a jury in the Federal District Court of Delaware decided the CoreValve infringes on transcatheter device patents held by Edwards Lifesciences and awarded Edwards $394 million in damages. Medtronic said it intends to appeal the decision.
Edwards Lifesciences challenged CoreValve with similar patent litigation in Europe, where both devices were first approved. Medtronic recently won a case in Germany, where an injunction against its sale has now lifted.
Very Positive Clinical Results
The Extreme Risk Study met its primary endpoint of death or major stroke at one year with a rate of 25.5 percent, which was 40.7 percent lower (p<0.0001) in patients treated with the CoreValve than was expected (based on a performance goal developed in partnership with the FDA). At one month, the rate of stroke was 2.4 percent, and it remained low over time with a one-year rate of 4.1 percent. Additionally, 75.6 percent of patients were alive at one-year. Contemporary results through the Continued Access Study, an extension of the U.S. pivotal trial, demonstrated even better survival and stroke performance.
“The low rates of stroke and valve leakage with the CoreValve System — two of the most concerning complications of valve replacement because they increase the risk of death and have a dramatic impact on quality of life — set a new standard for transcatheter valves,” said Jeffrey J. Popma, M.D., director of interventional cardiology at the Beth Israel Deaconess Medical Center, Boston, and co-principal investigator of the trial. “The CoreValve U.S. Pivotal Trial was rigorously designed and applied clinical best practices. The trial results have redefined optimal TAVR outcomes in the areas that matter most to physicians and their patients, and the results are especially remarkable given the complex medical conditions and extreme frailty of this population.”
In the U.S. pivotal trial, the CoreValve System also achieved exceptional hemodynamics, or blood flow, post-implant with results similar to the gold standard, surgical valves. Additionally, valve leakage (known as paravalvular leak or PVL) rates were low and decreased over time as the self-expanding valve conformed to the shape of a patient’s annulus — an improvement that has not been reported in other major TAVR studies.
CoreValve Offers Broad Range of Sizes
The CoreValve System was developed to serve the needs of the broadest range of patients with severe aortic stenosis. The FDA approved the entire CoreValve platform including the CoreValve Evolut 23 mm, and the CoreValve 26, 29 and 31 mm valves. With the broadest size range available, the CoreValve System is suitable for patients with native valves of nearly all sizes. Its self-expanding nitinol frame enables physicians to deliver the device to the diseased valve in a controlled manner, allowing for accurate placement. All valve sizes are delivered via the smallest (18 French, or 6 mm) TAVR delivery system available, making it possible to treat patients with difficult or small vasculature.
“The FDA approval of CoreValve System is important for U.S. heart teams as the CoreValve System will serve the broadest spectrum of aortic stenosis patients who are unable to undergo surgery,” said John Liddicoat, M.D., senior vice president, Medtronic, and president of the Medtronic Structural Heart Business. “By leveraging Medtronic’s history and expertise in bringing therapies to patients, we are supporting heart teams through training and education, imaging and patient evaluation programs that exemplify our safe and deliberate approach to patient access.”
Since obtaining CE (Conformité Européenne) mark in 2007, the CoreValve System has been implanted in more than 50,000 patients outside the United States.
For more information: www.corevalve.com