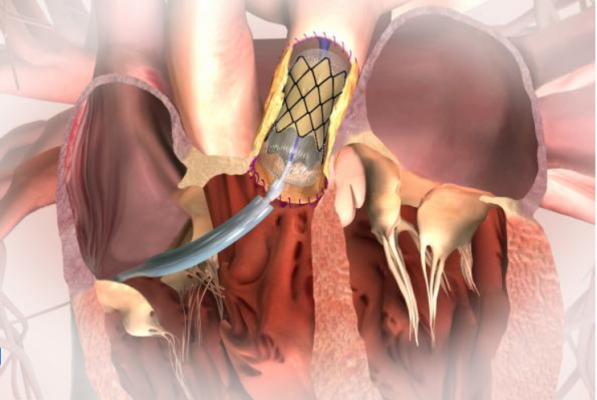
March 14, 2017 — The United States Food and Drug Administration (FDA) has expanded use of the Medtronic Melody Transcatheter Pulmonary Valve (TPV) for implantation in patients whose surgical bioprosthetic pulmonary heart valves have failed. Designed specifically for the pulmonic position, Melody TPV is the first transcatheter pulmonary valve to receive this approval in the U.S.
When a surgical valve degenerates over time, patients may require another valve replacement, which would involve undergoing another open-heart surgery. Intended to prolong the time between open-heart surgeries for patients with a dysfunctional right ventricular outflow tract (RVOT) conduit caused by CHD, the Melody TPV may now provide these patients with a minimally invasive treatment option.
“As the first commercially available transcatheter heart valve, the Melody TPV brought a breakthrough non-surgical option to treat failing pulmonary valve conduits,” Jeremy Asnes, M.D., associate professor of pediatric cardiology and director of the Congenital Cardiac Catheterization Laboratory at the Yale School of Medicine in New Haven, Conn. “Thousands of congenital patients globally have benefited from this therapy in the past decade. With this expanded indication, we can further reduce the need for obtrusive open-heart surgery and allow even more patients to benefit from this minimally invasive treatment option.”
During the procedure, the Melody TPV is placed inside a failing pulmonic surgical heart valve through the recently launched Ensemble II Delivery System, a low-profile, delivery catheter, specifically designed to deliver the Melody TPV.
The first transcatheter heart valve available anywhere in the world—and now implanted in more than 10,500 patients worldwide—the Melody TPV first received CE mark in September 2006 for the treatment of failing pulmonary valve conduits. It was introduced in the U.S. in 2010 following FDA approval for a humanitarian device exemption. The valve received full FDA approval in January 2015.
Over the last 10 years, clinical evidence from three Medtronic clinical studies has demonstrated the valve’s effectiveness in delaying the need for open-heart re-operation.
Related Content:
FDA Clears Sapien for Pulmonary Valve
Bioresorbable Pulmonary Valve Replacement May Enable Cardiovascular Regeneration
For more information: www.medtronic.com


 July 31, 2024
July 31, 2024 









