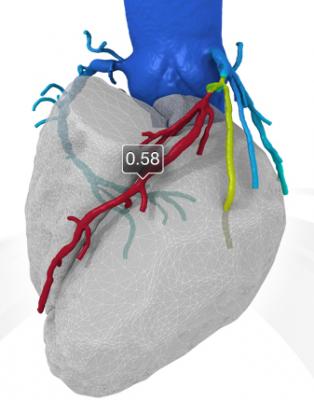
HeartFlow FFR-CT can noninvasively assess the hemodynamic impact of coronary lesions to avoid the need for an invasive angiogram.
July 6, 2017 — GE Healthcare and HeartFlow Inc. announced at the annual scientific meeting of the Society of Cardiovascular Computed Tomography (SCCT), that they have entered into a global collaboration agreement with the goal of increasing the clinical availability and adoption of HeartFlow fractional flow reserve computed tomography (FFR-CT), a proprietary technology that helps clinicians diagnose and treat patients with suspected coronary artery disease (CAD).
The collaboration will involve computed tomography (CT) scanners from GE Healthcare with HeartFlow FFR-CT, the first and only non-invasive technology that provides insight into both the extent of CAD and the impact of the disease on blood flow to the heart. FFR-CT is designed to enable clinicians to select a definitive, personalized treatment plan for each patient and reduce the need for additional invasive testing. The agreement will initially focus on the United States, with plans to expand into other markets in the future.
Clinicians diagnosing a patient with suspected CAD want to know as definitively as possible if the patient has a significant blockage in their coronary arteries and the hemodynamic impact of that blockage on blood flow to best determine which treatment pathway is appropriate (e.g., medical management, stenting or coronary artery bypass graft). With FFR-CT, data from a patient’s non-invasive coronary CT angiogram are securely uploaded from the hospital’s system to the cloud. Then, HeartFlow uses deep learning to create a personalized, digital 3-D model of the patient’s coronary arteries and uses powerful computer algorithms to solve millions of complex equations to simulate blood flow in the model and assess the impact of blockages on coronary blood flow. The results are provided to the patient’s clinician via a secure web interface to offer actionable information on the optimal course of treatment.
This is the second collaboration announced in 2017 with a major CT vendor. Siemens also began a FFR-CT collaboration with Heartflow, which was announced at the American College of Cardiology (ACC) in April.
Watch the VIDEO "Implementation and the Science Behind FFR-CT," an interview with James Min, M.D., Professor of Radiology and Medicine and Director of Dalio Institute of Cardiovascular Medicine, Weill Cornell, New York Presbyterian Hospital.
“GE has collaborated with HeartFlow over the last five years, and this agreement reinforces our joint commitment to patients worldwide,” said Scott Schubert, general manager, gobal premium CT, GE Healthcare. “Along with our industry-leading cardiac CT systems and clinical applications, GE can now offer HeartFlow FFR-CT as an option for healthcare providers who strive to deliver the highest standards in clinical care while reducing costs.”
“This agreement with GE will help bring our game-changing non-invasive technology into the mainstream of cardiac care at thousands of hospitals that are already using state-of-the-art CT systems from GE, which provide exceptional image quality,” said John H. Stevens, M.D., president and chief executive officer of HeartFlow. “By collaborating, we can ensure that HeartFlow FFR-CT can be easily integrated into existing CAD protocols and more readily transform the care of patients with suspected and potentially life-threatening CAD.”
Clinicians diagnosing a patient with suspected CAD want to know as definitively as possible if the patient has a significant blockage in their coronary arteries and the impact of that blockage on blood flow to best determine which treatment pathway is appropriate (e.g., medical management, stenting or coronary artery bypass graft).
With HeartFlow FFRct, data from a patient’s non-invasive coronary CT angiogram are securely uploaded from the hospital’s system to the cloud. Then, HeartFlow leverages deep learning to create a personalized, digital 3-D model of the patient’s coronary arteries and uses powerful computer algorithms to solve millions of complex equations to simulate blood flow in the model and assess the impact of blockages on coronary blood flow. The results are provided to the patient’s clinician via a secure web interface to offer actionable information on the optimal course of treatment.
GE brings to the collaboration its leading portfolio of cardiac CT solutions, including the Revolution family of CT scanners, with innovations including One-Beat Cardiac, SnapShot Freeze intelligent motion correction and high definition (HD) imaging with the industry’s highest cardiac spatial resolution. GE also offers
CardioGraphe, the world’s first dedicated cardiovascular CT system, a whole-heart coverage CT system that is affordable and accessible; and AW advanced clinical applications including CardIQ, VesselIQ and TAVR planning to enhance physician diagnostic accuracy and productivity.
Together, the combination of GE and HeartFlow technologies promises to become an important way to assist in diagnosing CAD and guiding appropriate treatment. This combination also could help reduce unnecessary and invasive diagnostic coronary angiography procedures, which can be associated with serious complications, such as bleeding, stroke and major blood vessel damage.[1]
Watch the VIDEO “Early U.S. Experience With FFR-CT in Evaluating ED Chest Pain Presentation.” A discussion with Simon Dixon, M.D., MBChB, on the use of fractional flow reserve-computed tomography (FFR-CT) to evaluate chest pain patients in the emergency departmenat Beaumont Hospital in Royal Oak, Mich.
Read the article "Clinical Applications of FFR-CT."
For more information: www.gehealthcare.com
References:
1. Douglas PS, DeBruyne B, Pontone G., Patel MR, et al. One-year outcomes of FFRCT-guided care in patients with suspected coronary disease: The PLATFORM Study. J Am Coll Cardiol. 2016;68(5),435-45.


 January 09, 2026
January 09, 2026 









