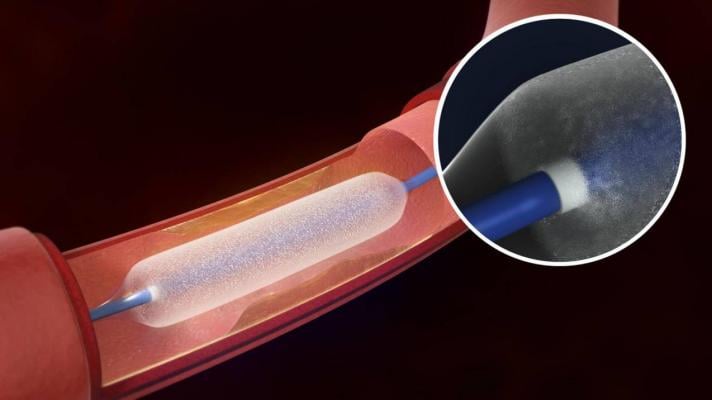
August 29, 2017 — C.R. Bard Inc. announced the Lutonix 035 Drug Coated Balloon PTA Catheter (DCB) has been granted premarket approval (PMA) by the U.S. Food and Drug Administration (FDA) for a new indication. With this approval, the device becomes the first and only drug-coated balloon that is FDA-approved as safe and effective in end-stage renal disease (ESRD) patients with stenotic lesions in dialysis arteriovenous (AV) fistulae.
This latest approval adds to the prior FDA indication of the Lutonix 035 for the treatment of superficial femoral artery (SFA) and popliteal artery disease.
The FDA approval was based on the results of the LUTONIX AV Clinical Trial, the first investigational device exemption (IDE) trial using drug-coated balloons in patients with stenotic lesions in AV fistulae. The follow-up results from randomized patients who were treated with the device demonstrated safety comparable to uncoated balloons.
The Lutonix 035 also demonstrated a sustained clinical benefit compared to conventional angioplasty through 12 months:
- Target lesion primary patency (TLPP) of 71.4 percent at 180 days, with superior results at 210 days (DCB 64.1 percent vs. percutaneous transluminal angioplasty [PTA] 52.5 percent);
- Fewer reinterventions compared to PTA at 6 months (31.3 percent);
- Nearly two (2) months more reintervention-free days (217 days vs. 163 days) compared to PTA;
- Improvement in TLPP through 12 months of 31.2 percent; and
- Freedom from primary safety event of 95 percent, indicating a consistent safety profile to PTA.
Globally, there are more than 2 million patients undergoing hemodialysis treatments¹ – each therapy session lasting approximately four hours and occurring up to three times per week – in a hospital or dialysis center.² To receive this therapy, many patients depend on an AV fistula³, which is the connection of an artery to a vein created by a vascular surgeon. For these patients, blockages created by repeated access or narrowing of the blood vessel (restenosis) are a common problem and hinder treatment. Some patients require up to eight reinterventions per year to maintain AV fistula function⁴, their lifeline for managing renal disease.
“For patients undergoing hemodialysis for kidney failure – who already spend a significant portion of their time undergoing dialysis and other treatments – repeated reinterventions to maintain AV access can be an added burden, with many patients returning as frequently as every other month,” said Scott O. Trerotola, M.D., Stanley Baum Professor of Radiology, associate chair and chief, interventional radiology, Perelman School of Medicine of the University of Pennsylvania and principal investigator of the LUTONIX AV Clinical Trial. “The Lutonix 035 DCB Catheter provides another option for physicians. It’s intended to offer patients with end-stage renal disease fewer interruptions in treatment and less time undergoing access maintenance, potentially leading to improved patient satisfaction and quality of life.”
The LUTONIX AV Clinical Trial included 285 subjects with lesion locations ranging from AV anastomoses at the wrist to the axillosubclavian junction at the shoulder. The trial design incorporated core laboratory evaluations on all patients, monitoring of all data points, independent Clinical Events Committee (CEC) adjudication and Data and Safety Monitoring Board (DSMB) review.
The two-year study is ongoing with additional endpoints at 18 and 24 months.
For more information: www.crbard.com
Read about the first FDA approvals for DCBs "Drug-eluting Balloons Enter the U.S. Market."
References
1. ESRD Patients in 2013: A Global Perspective – Fresenius Medical Care.
2. A to Z Health Guide: Dialysis – National Kidney Foundation.


 June 13, 2024
June 13, 2024 









