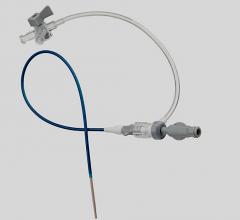
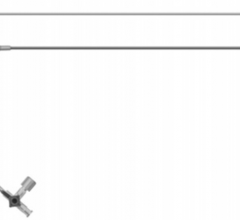
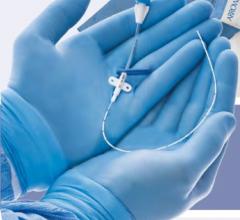
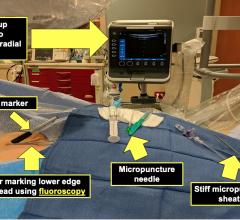
December 31, 2009 – A new micro-introducer kit launched this week features a stiffened introducer option. The AngioDynamics micro-introducers allow physicians to perform percutaneous introduction of a guide wire or catheter into the peripheral vasculature using a 21-gauge needle.
The kits were developed based on the physician feedback and include a needle, micro-introducer, and .018-inch guide wire. They include both stiffened and standard introducer options with various needle configurations, including super-sharp needles and echogenic tip options. The guide wire has a range of configurations, including stainless steel, nitinol, and tungsten. Tungsten-coiled tips allow for enhanced radiopacity, while the nitinol wire helps resist kinking.
The micro-introducer kits are available in 4 and 5 French sizes, in both standard and stiffened configurations, which facilitate smooth entry into difficult vessels. All feature a quarter-twist cam connection, which verifies a secure lock, and seamless dilator-to-sheath transitions.
For more information: www.angiodynamics.com

 June 10, 2020
June 10, 2020