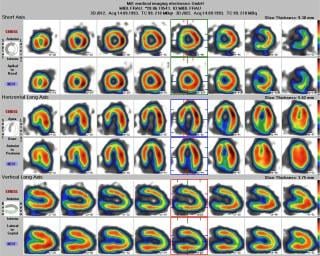
May 17, 2013 — The U.S. Food and Drug Administration (FDA) granted 510(k) clearance to Medical Imaging Electronics (MiE) and Nuclear Imaging Services (NIS) ECAT Scintron. It is the only upgrade path available to the Siemens ECAT 47, ECAT HR+, and ECAT Accel positron emission tomography (PET) systems. The ACSII and SUN workstation are removed from the system and replaced with new PC-based technology that provides new and existing users of the ECAT series PET systems access to faster processing speeds, new acquisition protocols and parts availability. With improved reliability and longevity, the ECAT Scintron redefines the life cycle of dedicated PET systems in the U.S. market.
The Scintron unit is always a part of a MiE system and can also be upgraded to any existing system. It takes on all processes of the scanner — data acquiring, processing, viewing as well as gantry controlling, calibrating and servicing.
For more information: www.mieamerica.com

 November 17, 2025
November 17, 2025 





![Phase III clinical trial of [18F]flurpiridaz PET diagnostic radiopharmaceutical meets co-primary endpoints for detecting Coronary Artery Disease (CAD)](/sites/default/files/styles/content_feed_medium/public/Screen%20Shot%202022-09-13%20at%203.30.13%20PM.png?itok=2w6OoNd6)
