December 12, 2014 — Siemens Healthcare presented two new clinical applications for angiography at the 2014 Radiological Society of North America (RSNA) annual meeting. The syngo Dyna4D software enables time-resolved 3-D imaging in angiography, permitting 3-D visualization of blood vessel volume as well as blood flow. The syngo DynaCT SMART algorithm allows the clinician to remove metal artifacts from patient medical images, potentially enabling the detection of complications such as hemorrhaging that occur in the vicinity of metallic objects contained within the patient’s body.
Syngo Dyna4D
In interventional radiology and neuroradiology, obtaining the best possible diagnosis is central to a therapy’s success because it allows precise treatment planning and execution. For these procedures, Siemens has developed syngoDyna4D angiography software, which unlike current 3-D image acquisition methods uses a modified protocol (“3D+t”) that enables the user to combine spatial and temporal resolution. This allows the clinician to track the passage of contrast medium in real time and see precisely how quickly and to what extent the patient’s vessels are filled. With this ability, the clinician can more precisely tailor therapy.
syngo DynaCT SMART
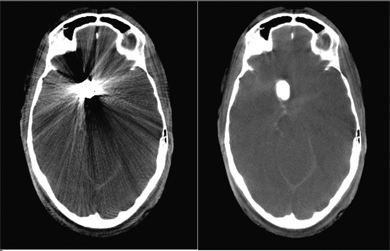
Metal objects in the patient’s body, including surgical clips or coils used in aneurysm therapy, cause massive streak metal artifacts in computed tomography (CT) or CT-like imaging as delivered using traditional syngo DynaCT. Because these streaks interfere with efforts to analyze areas of the body located near these metal objects, clinicians lack important information immediately following implantation that can help them identify complications such as hemorrhaging that can occur near the metal objects.
Siemens’ syngo DynaCT SMART algorithm removes metal streak artifacts from the image and enables visualization of regions near the metal for diagnostic purposes. The improved information content of the images helps to reduce the likelihood of errors and potentially the need for additional post-operative exams and/or hospital readmission.
Syngo Dyna4D and syngo DynaCT SMART will be available for Siemens’ Artis angiography systems with the new “Pure” platform for Artis zee, Artis Q, and Artis Q.zen. Both applications are currently U.S. Food and Drug Administration (FDA) 510(k)-pending.
For more information: www.siemens.com


 October 24, 2025
October 24, 2025 









