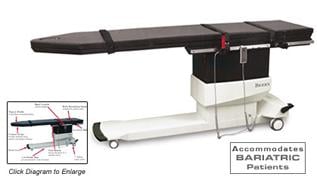
The Biodex Surgical C-Arm Table - 846 features a free-float X-Y tabletop design allowing for quick and comfortable patient positioning at an affordable price.
The large radiolucent area, motorized actuation of height and free-float tabletop make this table ideal for pain care and cardiovascular applications. The innovative contour of the tabletop design combines the advantages of square and contoured tabletops. Additional space is offered at the head-end of the table, while enabling the user to get the C-Arm close to the cervical spine for greater image resolution. The 500-lb patient capacity accommodates bariatric patients.
A portable hand-held controller can be positioned for convenient access from any point around the table, and hangs on an accessory rail when not in use. A push-button release for the free-float feature is located at the end of a handgrip that can be placed at any point on the OR accessory rail.
Designed for procedures where stability, access and precise, quiet, vibration-free positioning are essential. A cantilevered low attenuation carbon fiber tabletop accommodates portable or ceiling-suspended C-Arms. The radiolucent area is free of cross members allowing full fluoroscopic visualization and unobstructed C-Arm positioning. Functional design provides complete access with reduced radiation exposure to clinicians.
For more information: www.biodex.com


 October 24, 2025
October 24, 2025 









