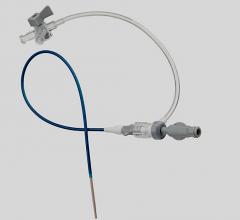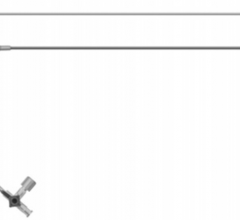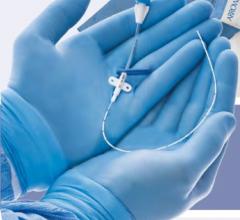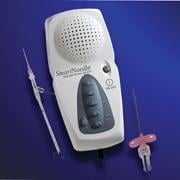
January 11, 2011 – A new vascular access system is available in the United States and Europe. The SmartNeedle Vascular Access System, by Vascular Solutions, consists of a hand-held monitor and one-time use needles designed to provide auditory ultrasound-guided access during catheterization procedures.
The Doppler guided needle access system was acquired from Escalon Medical in May 2010.
The reusable, hand-held monitor transmits a continuous wave Doppler signal, providing continuous auditory feedback to help locate and access the artery or vein quickly. If desired, the monitor can be covered with a disposable sterile bag for use in the sterile field.
Sterile, single-use needles range from 18G to 24G, with bare tip and sheathed IV options. Bare-tipped needles are available in 18G, 20G and 22G sizes, and consist of a detachable Doppler probe that is housed within the lumen of a standard sized introducer needle. IV sheathed needles, used when attempting to access a vessel with the intent of leaving a small catheter in place, are available in 20G, 22G, 24G and 26G sizes.
For more information: www.vascularsolutions.com

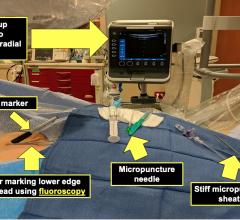
 June 10, 2020
June 10, 2020