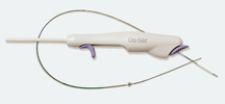
The On-Site vascular closure device is designed for precise and effective percutaneous arterial closure without leaving intra-arterial components behind that can potentially cause trauma.
Over-the-wire placement secures collagen precisely above the arteriotomy to achieve hemostasis. The precision closure device delivers an extravascular collagen sponge plug at the femoral arterial puncture site. Advanced Micropore sponge collagen provides a mechanical barrier within the tissue tract while enhancing the body’s natural healing process.
A temporary intra-arterial retractable disc is used to locate the arteriotomy, provide temporary hemostasis and ensure precise collagen placement. On-Site is compatible with standard length 5 F and 6 F procedural sheaths.


 October 07, 2025
October 07, 2025 









