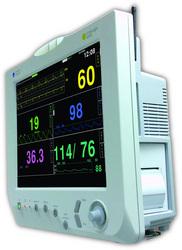
February 26, 2010 – The VitaLogik 6000 patient monitor was released this week by Mennen Medical. The preconfigured, multiparameter system has a 15-inch flat screen display, supported by an internal battery for uninterrupted vital signs' monitoring.
The VitaLogik 6000 has an extensive storage capability of full disclosure, charts, trends and events, in addition to the saved data of up to 10 discharged patients. It uses Mennen Medical's proprietary CIPAM (configurable isolated physiological acquisition module) technology, which is the company’s front end board, featuring multiple acquisition units for measuring various physiological parameters. It was recently recognized by Frost & Sullivan with "The European Patient Monitoring Technology Leadership of the Year Award 2009."
An integrated wireless local area network (LAN) solution has been developed specifically for the VitaLogik 6000, utilizing Mennen Medical's new propriety internal wireless card and based on the IEEE Compliance Standards 802.11b/g. The wireless network option for VitaLogik portable and bedside monitors extends the central station monitoring and automated vital sign collection beyond the bedside, because patient care can now be anywhere; by the bedside, in the hallway, during transportation or in auxiliary units. The new internal wireless network card will also be integrated into the portable VitaLogik 4000 series and is scheduled for release in the first quarter of 2010.
For more information: www.mennenmedical.com


 October 21, 2025
October 21, 2025 









