FEI released RemoteViz for Open Inventor, a software development framework for interactive…
…
September 24, 2014 — GE Healthcare announced U.S. Food and Drug Administration (FDA) 510(k)…
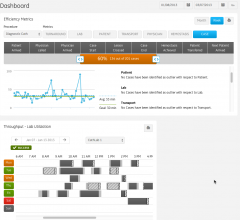
September 17, 2014 — GE Healthcare announced it has installed its Cath Lab Efficiency Manager at…
September 12, 2014 — Ecolab Healthcare announced the availability of the PD220 patient drape,…
September 11, 2014 — Medtronic announced the U.S. Food and Drug Administration (FDA) 510(k)…
September 8, 2014 — Esaote North America announced its new MyLab Six ultrasound system has…
September 8, 2014 — Eizo Inc. expanded its U.S. Food and Drug Administration (FDA) 510(k)-…
The U.S, Food and Drug Administration (FDA) has…
September 5, 2014 — CardiacAssist announced it has received 510(k) clearance from the U.S. Food…
CorMatrix Cardiovascular announced that it has received U.S. Food and Drug Administration (FDA)…
The easy-to-use online resource mHealth Roadmap includes information on implementation…
August 27, 2014 — Philips Healthcare announced it has received 510(k) clearance from the U.S.…
Cath Labs, interventional radiology suites and…
Fysicon received approval from the U.S. Food and Drug Administration (FDA) to market Fysicon…
Esaote is launching MyLab Six, a fast, accurate and highly flexible enhanced ultrasound system…
Esaote is launching MyLab Gamma, a best-in-class, highly portable “green” cardiovascular…
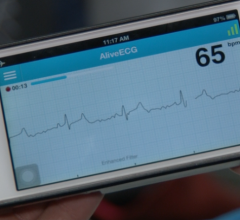
August 22, 2014 — AliveCor announced the U.S. Food and Drug Administration (FDA) has granted the…
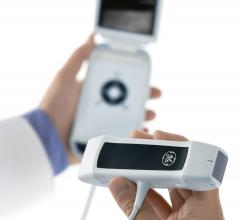
GE Healthcare introduces the Vscan with Dual Probe with the first of its kind 2-in-1 probe to…
August 20, 2014 — Advanced Ultrasound Electronics announced the availability of Sono-Wipes, a…

 September 25, 2014
September 25, 2014