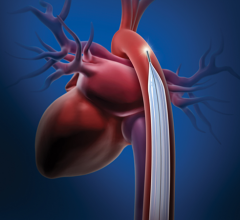
November 1, 2012 — InspireMD Inc. announced that its proprietary MGuard embolic protection stent (EPS) was shown to be significantly superior when compared to standard bare metal and drug-eluting stents in achieving complete ST resolution and restoring normal blood flow in a major study of 432 randomized patients undergoing emergency coronary intervention for potentially fatal heart attacks.

October 31, 2012 — Boston Scientific Corp. received CE mark approval for the Synergy everolimus-eluting platinum chromium (PtCr) coronary stent system featuring an ultra-thin abluminal (outer) bioabsorbable polymer coating.

Breast cancer patients who receive radiation treatment do not have a higher risk of long-term cardiac morbidity when compared to patients undergoing modified radical mastectomy (MRM), according to research presented at the American Society for Radiation Oncology’s (ASTRO’s) 54th Annual Meeting. This is the first study to document comprehensive, late cardiac outcomes 25 years after breast cancer treatment.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Maquet Cardiovascular received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and CE mark approval for its new Sensation Plus 7.5 French 40 cc intra-aortic balloon (IAB) catheter. This new larger-volume, fiber-optic IAB catheter will allow clinicians to provide a higher-efficacy IAB counterpulsation therapy “at the speed of light” to smaller patients.
W. L. Gore & Associates (Gore) responded to initial results reported in St. Jude Medical Inc.’s RESPECT clinical trial. The RESPECT study investigated whether transcatheter closure of patent foramen ovale (PFO) using St. Jude’s Amplatzer PFO Occluder device is safe and effective compared to best medical therapy in the prevention of recurrent cryptogenic stroke. Gore is concurrently conducting its Gore REDUCE Clinical Study using both the Gore Helex Septal Occluder and, as reported earlier this week, the new Gore Septal Occluder, in patients with PFO and a history of cryptogenic stroke or imaging-confirmed transient ischemic attack (TIA).

St. Jude Medical Inc., a global medical device company, announced that fractional flow reserve (FFR)-guided treatment using PressureWire was cost effective for coronary interventions when compared to the best available medical therapy. Cost utility analysis data from the FAME II trial was presented as a late-breaking clinical trial at the 24th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, sponsored by the Cardiovascular Research Foundation.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Elixir Medical Corp. announced enrollment completion of the 120-patient, pivotal clinical trial evaluating the safety and efficacy of the DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold System. The scaffold is designed to resorb in the body within one to two years after implantation and return the patients’ coronary vessel to de novo state.
The Philips IntelliSpace Portal is an advanced visualization solution designed to simplify the way radiologists work, think and care for patients by delivering on the promise of real-time radiology.
October 29, 2012 — At RSNA 2012, GE Healthcare will unveil innovations in healthcare IT, including enhancements to Centricity PACS (picture archiving and communication system) and PACS-IW. The PACS-IW system aligns advanced visualization, breast imaging and intelligent tools with a unified desktop.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Boston Scientific Corporation has received regulatory approval to market the Reliance 4-Front lead, its next generation implantable defibrillation lead now available in Europe and Asia. Defibrillation leads are insulated wires that connect an implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy defibrillator to the heart for treatment of heart failure and sudden cardiac arrest.
October 29, 2012 — Brainlab released Buzz Digital OR, a major step forward in information integration for the surgical suite. Buzz encompasses a vast spectrum, from pure DICOM viewing to complete digital operating room (OR) functionality, including video management and documentation.
Covidien announced final 12-month results from its DEFINITIVE LE (Determination of Effectiveness of SilverHawk/TurboHawk Peripheral Plaque Excision Systems for the Treatment of Infrainguinal Vessels/Lower Extremities) study.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
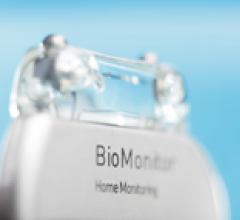
Biotronik began its European market release of BioMonitor, an implantable cardiac device designed for accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
October 25, 2012 — Lantheus Medical Imaging Inc. announced it has extended its contract with Nordion to supply molybdenum-99 (Mo-99) for use in its TechneLite (Technetium Tc-99m) generators.
October 25, 2012 — InterSystems Corp., a provider of software for healthcare interoperability, and eHealth Technologies, a provider of imaging solutions for electronic health records (EHR) and health information exchange (HIE) solutions, entered a partnership to provide single-click access to diagnostic-quality images via the InterSystems HealthShare strategic informatics platform.

 November 01, 2012
November 01, 2012