CardioCell LLC announced the treatment of its first patient in the University of Pennsylvania’s Phase IIa clinical trial for chronic heart failure (HF) of non-ischemic cardiomyopathy.
Turner MedTech introduced ClearShield at RSNA, which is lead-free, eco-friendly, utilizes no lead and complies with European Union RoHS requirements.
COR Medical Technologies (COR) introduces CORcare, a comprehensive instant outcome support system for rapid and accurate diagnosis, treatment, and follow-up management of patients with acute and chronic, common and rare diseases involving the cardiovascular system.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Children’s hospitals in the United States delivering the highest-quality care for children undergoing heart surgery also appear to provide care most efficiently at a low cost, according to research led by the University of Michigan and presented at the American Heart Association Scientific Sessions in Chicago.
Researchers at the Minneapolis Heart Institute Foundation have found that when care teams started following a new set of guidelines for certain patients recovering from cardiovascular surgery, hospital stays (and the associated costs) were reduced.
Breathing secondhand marijuana smoke could damage heart and blood vessels as much as secondhand cigarette smoke, according to preliminary research presented at the American Heart Association’s Scientific Sessions 2014.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
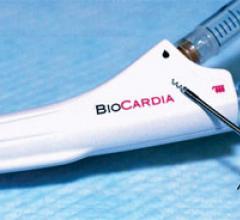
BioCardia Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s application to begin a Phase III clinical trial of its bone marrow-derived CardiAMP Therapy for heart failure. The clinical trial is a randomized, controlled, multi-center study of 250 patients evaluating CardiAMP Therapy at up to 40 clinical sites.
Most patients with implantable cardioverter defibrillators (ICDs) haven’t thought about device deactivation if they were to develop a serious illness from which they were not expected to recover.
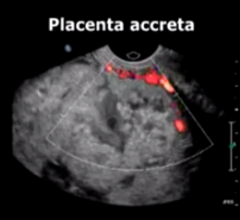
Researchers reported on a procedure that can preserve fertility and potentially save the lives of women with a serious pregnancy complication called placenta accreta. Results of the new study presented at the annual meeting of the Radiological Society of North America (RSNA) showed that placement of balloons in the main artery of the mother's pelvis prior to a Caesarean section protects against hemorrhage and is safe for both mother and baby.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
New research from the Minneapolis Heart Institute Foundation has uncovered a way to help interventional cardiologists better predict which patients are at higher risk for death following TAVR, allowing both patient and surgeon to discuss a potentially different treatment plan.
According to a new long-term study, diabetic patients with even mild coronary artery disease face the same relative risk for a heart attack or other major adverse heart events as diabetics with serious single-vessel obstructive disease.
Results from a new study indicate that the addition of mitral valve (MV) repair to coronary artery bypass grafting (CABG) did not result in significant benefit to the patient and was associated with increased risk of neurological events. Therefore, the routine addition of MV repair to CABG in patients with moderate ischemic mitral regurgitation (IMR) did not demonstrate a clinically meaningful advantage.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Fatigue, increased irritability and feeling demoralized may raise a healthy man or woman’s risk of first-time cardiovascular disease by 36 percent, according to a study led by researchers at Mount Sinai St. Luke’s and Mount Sinai Roosevelt hospitals. Study results were presented on Nov. 17 at the American Heart Association’s Scientific Sessions 2014 in Chicago.
Medi?Lynx Cardiac Monitoring LLC and MEDICALgorithmics will participate in a research project, investigating the impact of lifestyle modification on ablation outcome in atrial fibrillation (ISOLATE). The research is financed by Texas Cardiac Arrhythmia Research Foundation. The PocketECG cardiac monitoring system will be used for collecting and analyzing the ECG data in the project
Covidien announced that it received U.S. Food and Drug Administration 510(k) clearance for its Fortrex over-the-wire (OTW) percutaneous transluminal angioplasty (PTA) balloon catheter.

 December 09, 2014
December 09, 2014