June 23, 2025 — GE HealthCare’s commitment to advancing precision care in cardiology through its molecular imaging ...

Despite advancements in ventricular arrhythmia (VA) screening, numerous high-profile athlete cases have highlighted the ...
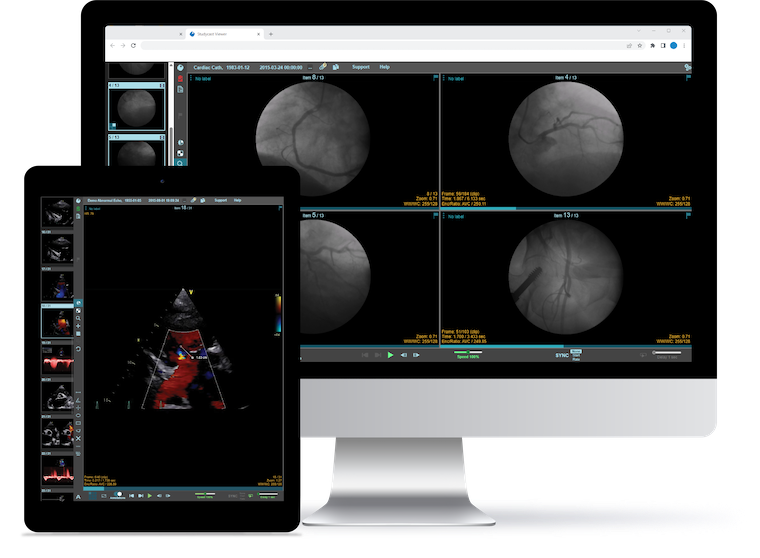
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
June 16, 2025 – Penumbra, Inc. recently announced the completion of enrollment in the STORM-PE clinical trial. This ...
June 13, 2025 — An international study has shown that targeted online education on atrial fibrillation (AF) for health ...
June 11, 2025 — MorningStar Laboratories, LLC, a developer of precision diagnostic tests that address unmet clinical ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
June 04, 2025 — HeartSciences Inc. has announced that the U.S. Food and Drug Administration has granted Breakthrough ...
June 12, 2025 — Viz.ai recently announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
June 12, 2025 – The American Society of Echocardiography (ASE) and Cardiovascular Research Foundation (CRF) have ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
June 12, 2025 — GE HealthCare has announced the combination of GE HealthCare’s proprietary features and algorithms with ...
June 11, 2025 — Bayer and the Broad Institute have have extended their research collaboration of 10 years by an ...

As healthcare systems continue to shift toward value-based care and proactive disease management, remote monitoring has ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 6, 2025 – A large retrospective review of more than 100,000 patients found the use of glucagon-like peptide-1 ...
June 5, 2025 – Penumbra, Inc. has announced the U.S. Food and Drug Administration (FDA) clearance and launch of the Ruby ...
June 4, 2025 — A new study published in The Annals of Thoracic Surgery, a journal from The Society of Thoracic Surgeons ...

 June 23, 2025
June 23, 2025