May 16, 2011 – In a coronary stent patent lawsuit, a jury said Cordis owes Boston Scientific approximately $19.5 million.
May 16, 2011 - The first patient was recently enrolled in the INOVATE-HF (INcrease Of VAgal TonE in Heart Failure) clinical study to determine the safety and efficacy of BioControl Medical’s CardioFit system. The implantable electrical stimulation device is designed to improve heart function in patients with congestive heart failure (HF). The first patient was enrolled at Northwest Texas Heart Hospital, Amarillo, Texas, by study investigator Suresh Neelagaru, M.D.
Michael Ringold, M.D., an interventional radiologist at St. Luke’s Hospital in Bethlehem, Pa., shared a case in which he used GE Healthcare’s Innova 4100 IQ interventional imaging system to perform dual embolizations on a 56-year-old man with liver cancer.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 11, 2011 - Micell Technologies announced that positive preclinical data will be presented at the EuroPCR conference regarding the MiStent Drug-Eluting Coronary Stent System (MiStent DES), an ultra-thin, advanced alloy drug-eluting stent distinguished by a rapid-absorbing drug/polymer coating formulation. The data will be presented in Paris, France on May 18, 2011 in a presentation titled "MiStent DES: A Novel Third Generation DES with a Fully-absorbable Coating and Enhanced Drug Delivery Capabilities."
Virginia, Minn. – Every day, Jack Luzovich steps on a special scale that helps keep him in his northern Minnesota home, rather than the hospital. At age 61, he is fighting the debilitating symptoms of congestive heart failure. Last summer, his health hit a low point. “I was retaining fluid and my weight was going up and down,” he remembers. “I couldn’t walk more than 10 to 15 feet. I was on oxygen, jaundiced and had almost no kidney function.” That’s when Jack received a telemonitoring scale from the Essentia Health Heart Failure Program (formerly St. Mary’s Duluth Clinic Heart Failure Program). The device records his weight and asks him questions about his health. It transmits that vital information to a cardiac nurse, who can make changes to his medications and track his condition on a daily basis.
Aortic dissection is rare, but when it occurs, a patient’s prognosis can be poor, even with timely medical diagnosis and treatment. It is the most frequently diagnosed lethal condition of the aorta. In the past, a type A dissection had been treated with surgery and a type B dissection with drugs to lower blood pressure and heart rate. Another option, thoracic endovascular aortic repair (TEVAR), where a stent-graft is placed inside the aorta, was introduced in 1999. This procedure restores the normal aortic anatomy to prevent aortic expansion or rupture. A study in the April issue of the Journal of Endovascular Therapy evaluates the performance of a specific stent-graft, the Relay thoracic stent-graft.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

May 11, 2011 – Terumo entered into a purchase agreement to acquire Harvest Technologies Corp, which is working to commercialize the world's first point-of-care stem cell therapy.
May 11, 2011 -The American Society of Echocardiography (ASE) will host its 22nd Annual Scientific Sessions: The Patient the Image: The Role of Cardiovascular Ultrasound in Clinical Decision Making (ASE 2011), June 11-14 at the Palais des Congres de Montreal in Montreal, Quebec, Canada.
May 10, 2011 – The Impella percutaneously deployed ventricular assist device showed an overall average hospital charge savings when compared to the standard-of-care intra-aortic balloon pump (IABP). The economic analyses is based on data from the PROTECT II study, which compares Impella to IABP hemodynamic support.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
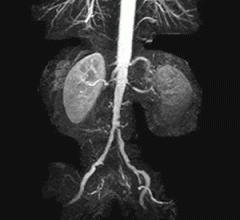
May 10, 2011 – Patients suffering from blockages of the arteries to the kidneys now have access to a new stenting treatment option with the launch of Cook Medical’s Formula Renal Balloon-Expandable Stent at the Society for Cardiovascular Angioplasty and Intervention (SCAI) 2011 Scientific Sessions. Commercial availability of Formula follows pre-market approval by the U.S. Food and Drug Administration (FDA) earlier this year for use in patients with atherosclerotic disease of the renal arteries.
May 10, 2011 – A simple blood test may help prevent a serious complication associated with a contrast agent commonly used in MRI exams, according to a study published in the July issue of Radiology.
May 10, 2011 – Thirty Society of Nuclear Medicine (SNM) members visited Capitol Hill on May 2 to meet with congressional offices on a variety of issues facing the nuclear medicine and molecular imaging community. Forty-eight meetings were held with staff members from key congressional committees and from the local districts of the SNM members.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 10, 2011 – The world's smallest and thinnest high-energy cardiac resynchronization therapy defibrillators (CRT-Ds) and implantable cardioverter defibrillators (ICDs) to treat heart failure and sudden cardiac death and offer excellent longevity were launched this week in Europe. Boston Scientific Corp. announced the launch and first implants of its Energen and Punctua CRT-Ds and ICDs in Europe and other international markets.
May 10, 2011 – In a “real-world” test of treatments for an overly thickened heart muscle, the use of alcohol injections to shrink the unwanted tissue appears to be significantly safer and less expensive than open-heart surgery to cut it away, according to a study presented today at the Society for Cardiovascular Angiography and Interventions (SCAI) 2011 Scientific Sessions.
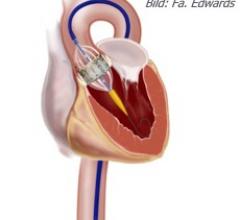
May 9, 2011 — John Webb, M.D., FSCAI, has traveled the globe teaching fellow physicians how to repair or replace faulty heart valves using minimally invasive techniques. In a keynote Founders’ Lecture delivered at the Society for Cardiovascular Angiography and Interventions (SCAI) 2011 Scientific Sessions last week, he recount the challenges that arose in developing valve therapies that avoid open-chest surgery. Instead, interventional cardiologists use slender tubes, or catheters, that are threaded into the heart through blood vessels.


 May 16, 2011
May 16, 2011