Medtronic Inc. announced it has received CE mark and will begin the European launch of the Attain Performa portfolio of quadripolar leads.
At the American College of Cardiology (ACC) conference, GE Healthcare unveiled DoseMap to alert interventional cardiologists to patient radiation exposure during longer procedures.

Accumetrics Inc. announced the presentation of a series of important data that solidifies the clinical utility of platelet reactivity testing. Real world outcomes data in high-risk patients receiving stents, a cost effectiveness analysis, and validation of a therapeutic window continue to demonstrate platelet reactivity as a critical element for improving the quality of care for the millions of patients on antiplatelet therapies worldwide.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
AliveCor announced that its mobile Heart Monitor for iPhone is now available by prescription to enable patients to record their heart rhythm anytime, anywhere. The Heart Monitor, which makes home cardiac assessment quick and easy, is the first U.S. Food and Drug Administration (FDA)-cleared mobile device–based ECG monitor that is compatible with iPhone4 and 4S. The device was demonstrated at the ACC.13 annual meeting of the American College of Cardiology March 9 through March 11 in San Francisco.
Agfa HealthCare demonstrated its Impax CV Web+ solution at the American College of Cardiology's (ACC) 62nd Annual Scientific Session and Expo held in San Francisco from March 9-11. The technology transforms cardiovascular care delivery by providing physician access to cardiovascular images and reports regardless of location.
For better treatment planning during cardiac resynchronization therapy (CRT), Toshiba America Medical System Inc.’s Activation Imaging is the company’s latest addition to its 3-D Wall Motion Tracking software. Activation Imaging is an U.S. Food and Drug Administration (FDA)-cleared, proprietary technology available on the Aplio Artida cardiovascular ultrasound system.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Abbott announced positive long-term results for the company's innovative Absorb Bioresorbable Vascular Scaffold (BVS). Three-year results from 101 patients in the second stage of the ABSORB trial were presented at the 62nd Annual Scientific Session of the American College of Cardiology (ACC) in San Francisco. Absorb is commercially available in Europe as well as other international markets and is an investigational device in the United States.
March 14, 2013 — Researchers from Perelman School of Medicine at the University of Pennsylvania are showing in a small study that while niacin increased measured levels of HDL-C, it did not improve the functionality of HDL. This may provide an explanation for the failure of niacin to further reduce cardiovascular risk. These study results were reported at the 62nd annual scientific session of the American College of Cardiology in San Francisco.
March 14, 2013 — Lantheus Medical Imaging Inc. announced the U.S. Food and Drug Administration (FDA) has granted approval of a supplemental new drug application (sNDA) that allows Jubilant HollisterStier (JHS) to be a new manufacturing site for its ultrasound imaging agent, Definity Vial for (Perflutren Lipid Microsphere) Injectable Suspension.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

March 13, 2013 — St. Jude Medical Inc. announced the first patient implant in a new pivotal trial evaluating the company’s Amplatzer cardiac plug (ACP) for the prevention of stroke.
March 13, 2013 — CircuLite Inc. announced it has received conditional approval from the U.S. Food and Drug Administration (FDA) for an investigational device exemption (IDE) for its lead product, the Synergy circulatory support system, a minimally invasive device designed to reverse the symptoms of heart failure in ambulatory chronic heart failure patients.
http://www.thelancet.comAtherosclerosis is usually considered to be related to contemporary risk factors such as smoking, obesity and lack of exercise. However, researchers suggest that high prevalence of atherosclerosis in pre-modern humans may support the possibility of a more basic human predisposition to the disease.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Many physicians have long believed that the use of intravenous contrast agents for computed tomography (CT) scans can cause acute kidney injury. New Mayo Clinic research questions the strength of the causal link between the two. The findings from two tandem studies are published online in the journal Radiology.
Using a telemedicine system to engage people in underserved, urban communities to measure and report their blood pressure remotely — outside of the doctor’s office — appears to help them achieve blood pressure goals and improve adherence to lifestyle changes and medication recommendations. This is according to research being presented at the American College of Cardiology’s 62nd Annual Scientific Session. Overall, researchers say that just being in a system of care, with or without telemedicine, can result in important reductions in blood pressure.
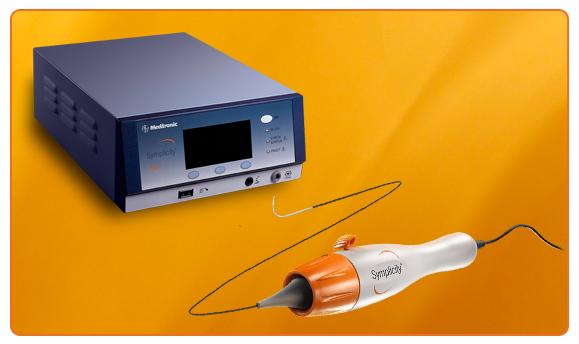
Medtronic Inc. announced the U.S. Food and Drug Administration (FDA) and the Centers for Medicare and Medicaid Services (CMS) have accepted the inclusion of the Symplicity renal denervation system for treatment-resistant hypertension in their parallel review program, which will allow CMS to begin consideration for national coverage determination while the FDA completes its review of safety and efficacy.

 March 18, 2013
March 18, 2013