One life-threatening complication of lung cancer surgery is the formation of blood clots in the lungs (also called pulmonary embolism, PE) or in the legs (also known as deep vein thrombosis, DVT). Together, they would be defined as venous thromboembolic events (VTE). Several presentations at AATS 2015 shed new light on this serious problem. In the first prospective study of its kind, the incidence of VTE was found to be higher than previously reported, with a 5.4 percent VTE-specific mortality rate. Of concern to clinicians, most events were asymptomatic and occurred after patients were discharged from the hospital. The second report highlights the importance of screening for VTEs, especially since the majority of lower extremity VTEs found after pneumonectomy would have gone undiagnosed and untreated without screening. The third report describes a risk assessment tool for VTEs that is applied for the first time to predict an individual’s risk of VTEs after lung cancer surgery, which can help clinicians decide whether prolonged anti-clotting therapy is warranted.
The Society for Cardiovascular Angiography and Interventions (SCAI) announced the following late-breaking clinical trials to be presented at its 2015 Scientific Sessions, May 6-9 in San Diego.
AstraZeneca announced that the U.S. Food and Drug Administration (FDA) has accepted a supplemental new drug application (sNDA) and granted Priority Review for Brilinta (ticagrelor) tablets for patients with a history of heart attack. The sNDA is based on the results of the PEGASUS-TIMI 54 study, a large-scale outcomes trial in more than 21,000 patients that investigated ticagrelor tablets plus low dose aspirin, compared to placebo plus low dose aspirin, for the chronic secondary prevention of atherothrombotic events in patients who had experienced a heart attack one to three years prior to study enrollment. The Prescription Drug User Fee Act goal date will be in the third quarter of 2015.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Two global trials have found that the addition of the Solitaire device stent thrombectomy procedure to current pharmaceutical treatment significantly reduced disability in patients suffering stroke. Results of the trials were published online in The New England Journal of Medicine (NEJM) and presented at the European Stroke Organization Conference; they confirmed the findings of three previous trials also published in NEJM.
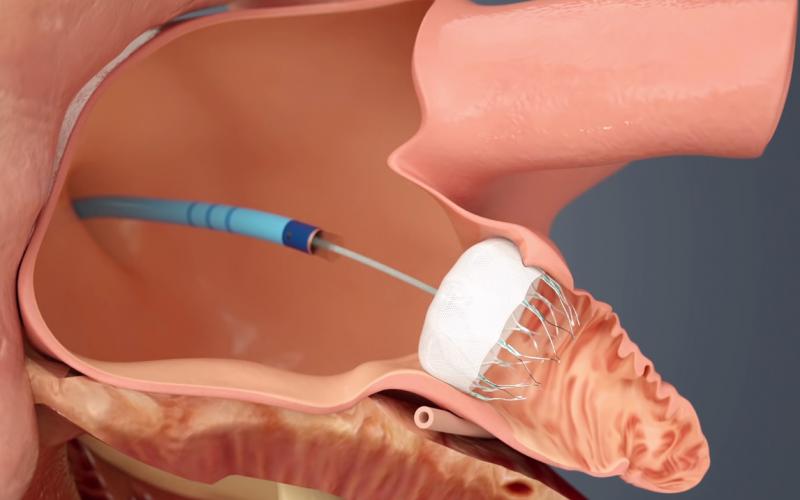
The long awaited U.S. Food and Drug Administration (FDA) approval of the first transcatheter left atrial appendage (LAA) occluder in March is seen by many cardiologists as a disruptive technology in the management of stroke risk in some atrial fibrillation (AF) patients. The FDA approval of Boston Scientific’s Watchman device offers a new stroke risk reduction option for high-risk patients with non-valvular AF who are seeking an alternative to long-term warfarin anticoagulant therapy.
Kaiba was just a newborn when he turned blue because his little lungs weren’t getting the oxygen they needed. Garrett spent the first year of his life in hospital beds tethered to a ventilator, being fed through his veins because his body was too sick to absorb food. Baby Ian’s heart stopped before he was even six months old.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

With higher deductibles and more price-conscious healthcare consumers, as a result of the Affordable Care Act, improving the performance, comfort and speed of MRI scans can be an important strategy for imaging centers that must compete by offering the best patient care and experience.
COR Medical Technologies (COR) announced that Gregg W. Stone, M.D. has been appointed to its Senior Editorial Board.
A key trial evaluating the Boston Scientific Eluvia drug-eluting vascular stent system met its primary endpoint, with more than 94 percent of the lesions treated for peripheral arterial disease (PAD) remaining open at nine months post implantation. This was accompanied by a target lesion revascularization rate (TLR) of less than four percent.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Several futuristic technologies and their potential impact on healthcare were discussed during the Future of Cardiovascular Medicine track sessions at the American College of Cardiology (ACC) 2015 annual meeting. Among these were use of big data, the integration of smartphones and wearable devices into patient care, robots in the cath lab, 3-D printing and how technology will help lower the cost of clinical trials for drugs and devices and speed their introduction to market.

The Medicines Company announced that the U.S. Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee (CRDAC) voted 9 - 2 with one abstention to recommend approval of the investigational intravenous antiplatelet agent cangrelor as an adjunct to percutaneous coronary intervention (PCI). Cangrelor is indicated for reducing the risk of periprocedural thrombotic events such as myocardial infarction (MI), stent thrombosis (ST) and ischemia-driven revascularization.
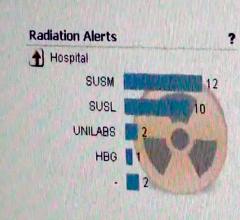
The American College of Radiology (ACR) and American Association of Physicists in Medicine (AAPM) are offering a new resource document for physicians and medical physicists serving as radiation safety officers at medical facilities. The ACR-AAPM Radiation Safety Officer Resources was developed jointly by medical physicists from both associations.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Researchers from Johns Hopkins performing sophisticated motion studies of heart magnetic resonance imaging (MRI) scans have found that specific altered function in the left atrium may signal stroke risk in those with atrial fibrillation and those without it.
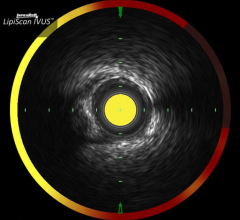
Infraredx Inc. announced the enrollment of 1,000 patients in the Lipid-Rich Plaque (LRP) Study. The LRP Study is a prospective, multi-center clinical trial designed to identify a correlation between lipid-rich plaques detected by Infraredx’s TVC Imaging System and the occurrence of a cardiac event within two years.
Biotronik announced that the U.S. Food and Drug Administration (FDA) has approved its DX line of implantable cardioverter defibrillators (ICDs) that can deliver ultra-high energy on the first shock. Also included in the approval response from the FDA is the latest generation of defibrillator devices for patients with complex heart rhythm conditions.

 May 01, 2015
May 01, 2015