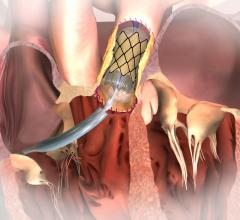
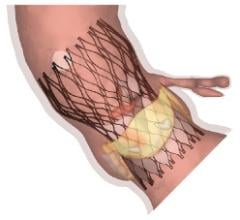
Medtronic revealed new one-year clinical data showing that transcatheter aortic valve replacement (TAVR) with the self-expanding CoreValve System offers advantages in survival and safety compared to surgical aortic valve replacement (SAVR) in high risk aortic stenosis patients who have previously undergone coronary artery bypass grafting (CABG) surgery.
St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval of the FlexAbility Ablation Catheter, a new ablation technology used by electrophysiologists for the treatment of cardiac arrhythmias.
The Diagnostics Division of Siemens Healthcare unveiled its latest point-of-care acute care analyzer for the management of patients with cardiovascular disease symptoms at Medlab in Arab Health 2015, Dubai, Jan. 26-29. The company’s Stratus CS 200 Acute Care Diagnostic System is designed to enhance simple operation and instrument management at the point of care to help facilitate rapid decision-making and optimal patient care.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A tool designed to assess what interferes with acute heart failure patients' ability to care for themselves after hospital discharge holds promise for improving patient outcomes and reducing readmissions to the hospital. The patient survey, designed by researchers at Vanderbilt University, was published online in Annals of Emergency Medicine, along with patient responses that shed light on the non-medical issues that limit patients' ability to care for themselves.
Neovasc Inc. has commenced a public offering in the United States of 8 million shares of the company to raise money for upcoming U.S. clinical trials and development of its transcatheter mitral valve and treatment system for refractory angina.
Maquet Medical Systems USA announced an agreement to serve as the exclusive U.S. distributor of InterValve Inc.'s V8 Aortic Valvuloplasty Balloon Catheter.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
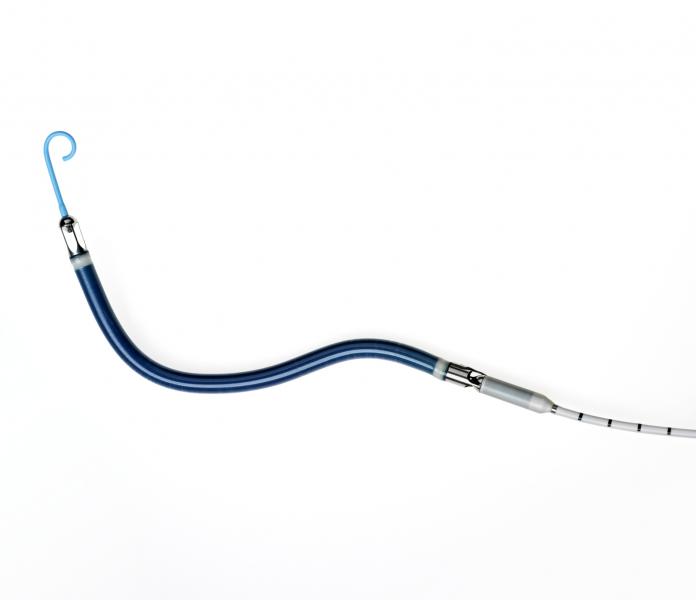
Abiomed Inc. said the Impella RP (Right Percutaneous) system has received U.S. Food and Drug Administration (FDA) approval under a humanitarian device exemption (HDE). This is the first percutaneous single access heart pump designed for right heart support to receive FDA approval. Abiomed completed the HDE submission for the Impella RP in September 2014 following the completion of the RECOVER RIGHT study.
The U.S. Food and Drug Administration (FDA) has granted approval of Medtronic’s premarket approval application (PMA) for the Melody Transcatheter Pulmonary Valve (TPV) and its Ensemble Transcatheter Valve Delivery System. This allows wider use of the valve in patients born with congenital heart defects that often require multiple open-heart surgeries. The valve is designed to reduce the number of open surgeries and expand the time between them.
NewYork-Presbyterian Hospital/Columbia University Medical Center is one of a select group of medical centers participating in a clinical trial of a new device for advanced heart failure patients, the Thoratec HeartMate III Left Ventricular Assist System (LVAS).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
ECRI Institute has released an updated comparison on cardiac resuscitators used during cardiopulmonary resuscitation (CPR).

Health and Human Services Secretary Sylvia M. Burwell announced measurable goals and a timeline to move the Medicare program and the healthcare system at large toward paying providers based on the quality, rather than the quantity of care they give patients. The announcement was made in a meeting with nearly two dozen leaders representing consumers, insurers, providers and business leaders.

Three-dimensional (3-D) printing can effectively create a biodegradable tracheal segment containing a patient’s own cells for use in complex tracheal reconstruction, according to a proof of concept study abstract released at the 51st Annual Meeting of The Society of Thoracic Surgeons.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
CytoSorbents Corporation announced that it has submitted an Investigational Device Exemption (IDE) application to the U.S. Food and Drug Administration (FDA) to conduct its proposed clinical trial using CytoSorb intra-operatively in patients undergoing complex cardiac surgery requiring the use of a heart-lung machine.
Ortho-Clinical Diagnostics Inc. announced the nationwide availability to hospitals of the Nephrocheck Test System designed to help healthcare providers identify patients at risk of developing moderate or severe acute kidney injury (AKI) within 12 hours of patient assessment.
FEops announced the closing of a €1.3 million (~$1.9 million USD) series A financing round, led by Capricorn Venture Partners and PMV. The funding will be used to support the launch of FEops’ first product, TAVIguide, in key markets in Europe and the United States.


 January 29, 2015
January 29, 2015