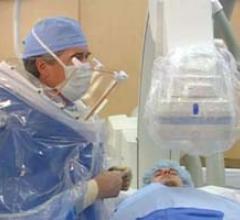
To prevent physician fatigue and back pain associated with wearing protective lead aprons in the cath lab, the ZeroGravity Radiation Protection System offers increased radiation protection without weighing down the user. Using a ceiling suspended gantry system, no weight is placed on the body of the interventionalist and it allows for smooth movement along the X, Y, Z axis.
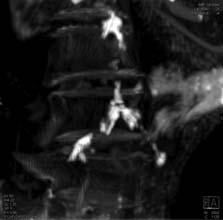
A U.S.-Egyptian research team has uncovered the earliest documented case of coronary atherosclerosis in a princess who died in her early 40s and lived between 1580 and 1550 B.C.
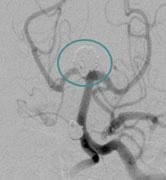
April 8, 2011 – The U.S. Food and Drug Administration (FDA) has approved an aneurysm treatment system as a Humanitarian Use Device (HDE). NeuroVasx’s cPAX Aneurysm Treatment System can be used to treat large, giant and wide-neck cerebral aneurysms, which are typically the most difficult to treat. The HDE allows for the treatment of up to 4,000 patients per year in the United States.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The U.S. Food and Drug Administration (FDA) this week released its February list of device and technology premarket approvals (PMA), product development protocols (PDP), supplement and notice decisions. To review the listing, visit: www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/ucm250089.htm
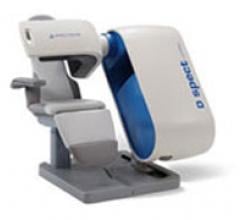
April 8, 2011 – Spectrum Dynamics has installed the second D-SPECT (single photon emission computed tomography) system using cadmium zinc telluride (CZT) nuclear imaging detectors. The new detectors enable a new generation of high resolution, low-dose nuclear cameras.
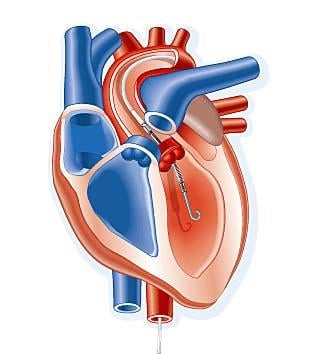
A transcatheter ventricular assist device performed better than intra-aortic balloon pumps (IABP), with a 21 percent reduction in major adverse events at 90 days.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 8, 2011 – At the American College of Cardiology (ACC) Annual Scientific Meeting in New Orleans, Siemens Healthcare demonstrated how users of nuclear imaging systems can use its IQ•SPECT to help reduce the length of imaging protocols.
April 7, 2011 – Primary results from the RADAR Phase 2b clinical trial were announced at the American College of Cardiology (ACC) 2011 60th Annual Scientific Session. The trial looked at Regado Bioscience’s Reg1 anticoagulation system.
April 7, 2011 – New study results have evaluated the potential inhibitory effects of certain proton pump inhibitors (PPIs) on clopidogrel (Plavix). The results showed that in healthy subjects, clopidogrel's active metabolite and inhibition of platelet function were reduced less by the co-administration of dexlansoprazole (Dexilant) or lansoprazole, rather than esomeprazole.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 7, 2011 – Data from two phase 3 trials studying a drug for high-cholesterol patients who are on lipid-lowering therapy were presented at American College of Cardiology’s (ACC) 60th Annual Scientific Session. The studies investigated mipomersen, from Genzyme, a subsidiary of sanofi-aventis, and Isis Pharmaceuticals.

April 7, 2011 — The American College of Cardiology (ACC) 2011 in New Orleans this week offered an expo floor filled with hundreds of cardiovascular products. The following five technologies stood out as my editor’s choice for innovation that addresses needs in cardiology. Immediate, Interactive ECGs on the iPad
April 7, 2011 – Esaote International NV will acquire 3mensio Medical Imaging BV in order to expand its role in healthcare information technology (IT). Following this acquisition, Pie Medical Imaging BV (PMI), a group company of the Esaote group, and 3mensio have announced that they have joined their business activities.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 7, 2011 – The first digital broadband magnetic resonance imaging (MRI) scanner has been cleared in the United States. Previously available in Europe, Canada and Japan, the Philips Ingenia MRI system received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
April 7, 2011 – At the recent American College of Cardiology (ACC) Annual Scientific Session in New Orleans, Siemens showcased several ultrasound products. Among the systems highlighted were the 1.6 release of its Acuson SC2000 volume imaging ultrasound system and IN Focus Technology, derived from the Acuson Sequoia ultrasound system.
April 6, 2011 – Data from the EVEREST II study show that patients with significant mitral regurgitation (MR) continue to show benefits from treatment with a catheter-based valve-clip system at one year out to two years. The study investigated Abbott’s MitraClip, which also showed improvements in heart function and reductions in symptoms.

 April 08, 2011
April 08, 2011