Medtronic’s Endeavor Zotarolimus-Eluting Coronary Stent System is engineered for the treatment of coronary artery disease. Endeavor uses the Driver bare metal cobalt alloy stent platform and the drug zotarolimus along with the proprietary, biocompatible drug delivery polymer.
Edwards Lifesciences Corp. has rolled out a next-generation transfemoral delivery system for the Edwards SAPIEN ...
May 13, 2008 – Spectranetics’ excimer laser sheath safely and effectively assists removal of pacing and defibrillator leads, according to the study, “Large, Single-catheter, Single-operator Experience with Transvenous Lead Extraction: Outcomes and Changing Indications,” featured in the April issue of HeartRhythm.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
GE Healthcare launched its new mobile Vivid S5 cardiovascular ultrasound system, created for multiple care areas ...
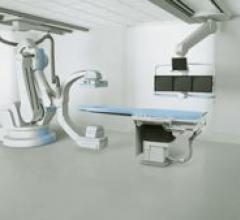
Siemens received FDA 510(k) clearance for Artis zeego, a robotic-assisted positioning capability for interventions in ...
May 13, 2008 – Echoserve launched an online portal designed to facilitate the research, purchase and sale of diagnostic ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Textronics Inc. received FDA clearance to market its textile-based ECG Electrode for use in general electrocardiograph monitoring and recording procedures, offering patients an alternative to adhesive electrodes and metal wristbands.
The FDA cleared CryoLife’s CryoValve SG pulmonary human heart valve processed with the company’s proprietary SynerGraft ...
May 9, 2008 – The Centers for Medicare and Medicaid Services (CMS) issued its final National Coverage Decision (NCD) to ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 9, 2008 - The FDA cleared Boston Scientific's ALTRUA family of pacemakers, a day after the European approval of ...
May 9, 2008 – Philips started shipping the upgrade Xcelera R2.2, a new version of the Xcelera multimodality cardiology ...
May 9, 2008 – The FDA approved Covidien’s contrast delivery system with radio-frequency identification (RFID) technology, integrating the technology to create a system that is designed to enhance patient safety by reducing the risk of medical errors in radiology departments.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
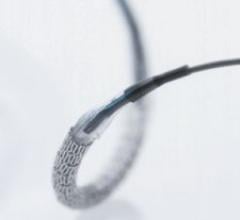
The Strada Carotid Guiding Sheath is designed specifically to provide easier and faster access to challenging carotid anatomy.
Philips offered new disposable adult ECG leadwire electrodes to help with infection control May 3-8 at the American Association of Critical-Care Nurses National Teaching Institute in Chicago.
May 9, 2008 – The FDA cleared the Strada Carotid Guiding Sheath, a flexible tube for physicians to deliver balloon ...

 May 12, 2008
May 12, 2008