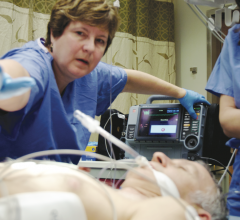
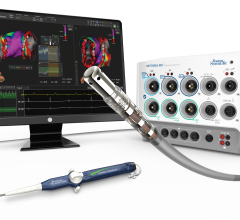
New cardiovascular device therapies for atrial fibrillation (AF) and heart failure (HF) are rapidly evolving with the ...
Increasing stomach fat – especially the “hidden fat” in your abdomen – is associated with newly identified and worsening heart disease risk factors, according to a study published recently in the Journal of the American College of Cardiology. These adverse changes in cardiovascular risk were evident over a relatively short period of time and persisted even after accounting for changes in body mass index (BMI) and waist circumference, two commonly used methods to estimate whether someone is a healthy weight or not.
Neuravi recently announced Conformité Européenne (CE) Mark approval and launch of the company’s newly available enhancements to the EmboTrap stent retriever platform, the EmboTrap II Revascularization Device.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
(Editor's note: links to newer information and video links from recent conferences were added to this article in ...
September 28, 2016 — In a recent study, a Yale Cancer Center team determined that men who received hormonal therapy for ...
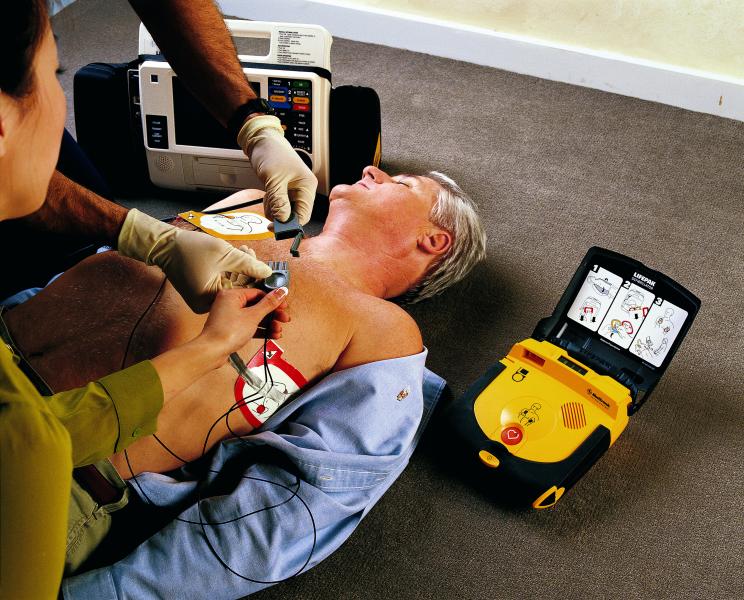
Automated external defibrillators (AED) are portable and lightweight devices used to deliver an electric shock through ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

September 28, 2016 — The Cardiovascular Research Foundation (CRF) included 11 late-breaking trials and 16 first report ...
Avinger Inc. recently announced the company has received an FSS Contract Award from the U.S. Department of Veterans Affairs (VA). The award establishes terms under which VA hospitals across the country may acquire Avinger’s Lumivascular portfolio of optical coherence tomography (OCT) image-guided catheters for their veterans hospitals.
In August, the European Heart Rhythm Association (EHRA) and EP Europace journal announced the release of the supplement to its ninth annual EHRA White Book, developed in partnership with Biotronik. The supplement was released just ahead of the 2016 European Society of Cardiology (ESC) Congress, Aug. 27-31 in Rome.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
BioTelemetry Inc. announced in late August that the company has received CE mark approval of its Holter analysis software. The software can be used either on a stand-alone basis or in combination with the company’s family of digital Holter recorders.
Nearly 2 out of 5 people with diabetes who could benefit from statin therapy to lower their risk of future heart attack, stroke and related death were not prescribed one, according to a research letter published in the Journal of the American College of Cardiology. The analysis also showed wide variation in statin use across cardiology practices included in the study.
C. R. Bard Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational Device Exemption (IDE) supplemental application to modify the primary endpoint to a six-month time point for the Lutonix 014 Drug Coated Balloon PTA Catheter (DCB). The Lutonix 014 device is currently the only DCB in an IDE clinical trial in the United States for treatment of arteries below the knee (BTK).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Smartphone communication among medical teams at different hospitals can significantly reduce the time it takes for heart attack patients to get lifesaving treatment after a hospital transfer, according to a research letter published in the Journal of the American College of Cardiology.
A Google search for heart conditions will now prominently display important questions patients should ask their doctor based on clinical guidelines developed by the American College of Cardiology.
Technology advances coupled with increased use of social media and personal devices could offer new possibilities for treating patients and improving outcomes, but new approaches must be rigorously evaluated, according to U.S. Food and Drug Administration Commissioner Robert M. Califf, M.D., MACC. Califf’s comments appeared in a column published in the Journal of the American College of Cardiology.

 September 29, 2016
September 29, 2016