September 8, 2014 — Eizo Inc. expanded its U.S. Food and Drug Administration (FDA) 510(k)-cleared large monitor system with the addition of the RadiForce LS580W monitor and LMM0802 large monitor manager for interventional radiology and surgical suites.
The clinical nurse educator working in the adjacent lab was called to assist and made the decision to use the LUCAS 2 mechanical chest compression device to provide automatic external chest compressions. The radiolucent carbon fiber backboard was placed under Vanessa’s shoulders and the piston/suction cup placed over her chest. Once started, the LUCAS 2 applied continuous chest compressions of at least 5 centimeters at a consistent rate of 100 or more compressions per minute.
After several years of trials and increasing clinical use, transcatheter aortic valve replacement (TAVR) has reached a tipping point. It is quickly becoming the standard of care for ill patients suffering from aortic valve stenosis who are not eligible for open heart surgery. Implemented successfully, TAVR procedures improve outcomes and quality of life for patients while reducing costs for hospitals.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Directional atherectomy is safe and effective as a frontline therapy for the treatment of peripheral arterial disease (PAD), according to a landmark Covidien study published online in the Journal of American College of Cardiology, Cardiovascular Interventions (JACC CI).
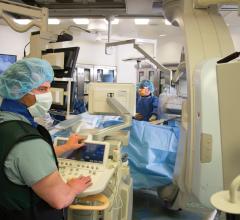
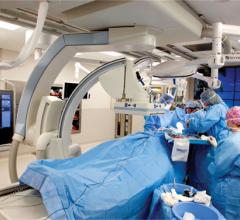
St. John Hospital currently performs about 2,000 percutaneous coronary and peripheral interventions and 400 open-heart surgeries, including minimally invasive and robotic surgery. With a goal to create a full structural heart program that answers its patients many needs, St. John Hospital recently created a hybrid operating room (OR). Among the challenges were deciding where to put the room, what procedures would be performed and what equipment would be necessary.
The U.S, Food and Drug Administration (FDA) has cleared MicroVention Inc.’s Low-Profile Visualized Intraluminal Support Device (LVIS and LVIS Jr.), which is a stent and delivery system used to treat certain brain aneurysms.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 5, 2014 — The number of uninsured is expected to decline by nearly half from 45 million in 2012 to 23 million by 2023 as a result of the coverage expansions associated with the Affordable Care Act (ACA), according to a report from the Centers for Medicare & Medicaid Services (CMS) Office of the Actuary. The report is published in Health Affairs.
September 5, 2014 — CardiacAssist announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new Protek Duo veno?venous cannula. The Protek Duo is intended for use as a single cannula for both venous drainage and reinfusion of blood via an internal jugular vein during extracorporeal life support (ECLS) procedures.
A new analysis of clinical trial participation in the largest ongoing observational study of U.S. heart attack patients has found participants are not representative of the larger patient base, according to a study led by Women’s College Hospital cardiologist Jay Udell.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
AirStrip, a provider of mobile healthcare applications, has raised $25 million in a strategic funding round led by the Gary & Mary West Health Investment Fund, Sequoia Capital and Wellcome Trust that includes some of the most influential leaders in healthcare.
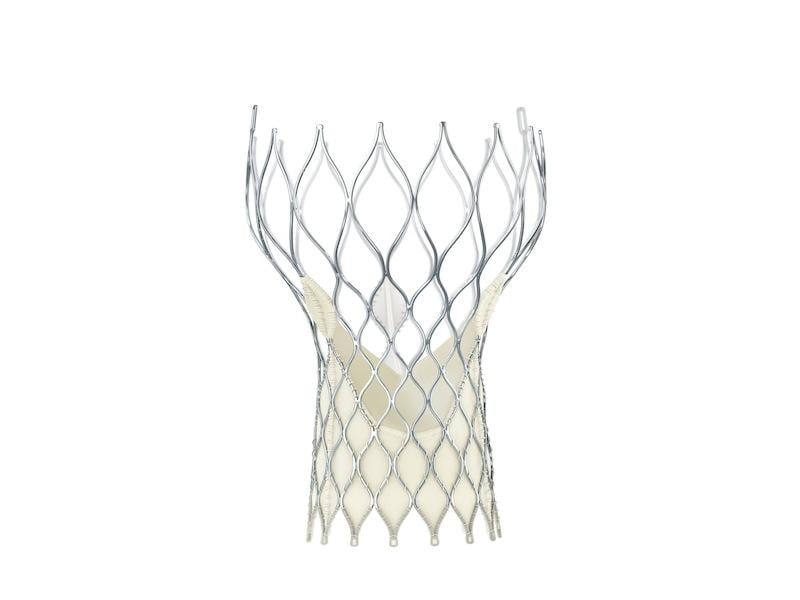
September 4, 2014 — Medtronic announced CE mark for the 23 mm CoreValve Evolut R system for transcatheter aortic valve implantation (TAVI). The novel self-expanding valve and 14 French equivalent delivery system offers new capabilities that advance valve performance and deliverability during the procedure, while providing the option to recapture and reposition the valve during deployment phase if needed.
September 4, 2014 — St. Jude Medical announced a new multicenter clinical trial has found that using the company’s fractional flow reserve (FFR) technology changed the course of treatment for more than one-fifth of patients suffering non-ST segment elevation myocardial infarction (NSTEMI) heart attacks.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Cardiovascular Cell Therapy Research Network (CCTRN), has selected Cytori Therapeutics, Inc. to supply adipose-derived regenerative cells (ADRCs) for a clinical trial aimed at evaluating the safety and feasibility of treating patients with Left Ventricular Assist Devices (LVADs).
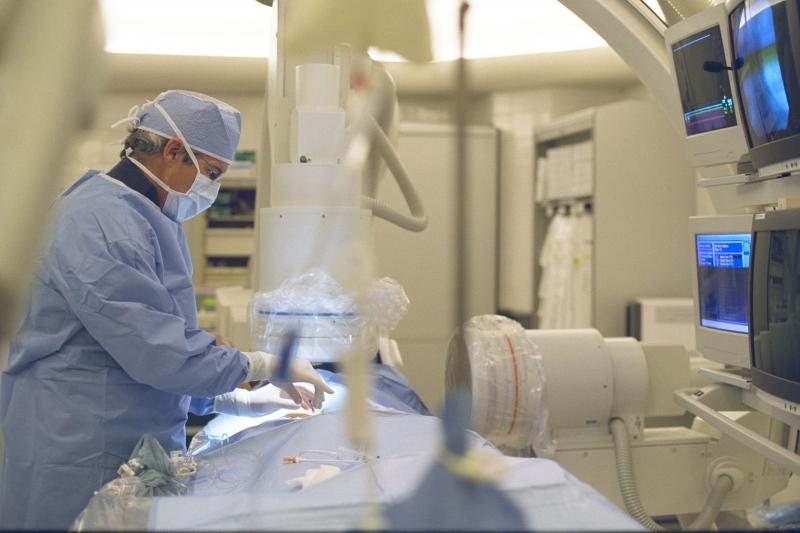
For the first time in the United States, doctors at Henry Ford Hospital used a minimally invasive procedure to replace a failing, hard-to-reach heart valve with a new one – and placed it just outside the heart.

Angiographic imaging system vendors have developed several new technologies to address emerging cath lab trends, including the need to reduce radiation dose, improve image quality and enable advanced procedural image guidance. All three of these points have become increasingly important as more complex procedures are attempted in interventional cardiology cath labs and hybrid ORs. These procedures include transcatheter aortic valve replacement (TAVR), mitral clip valve repairs, left atrial appendage (LAA) occlusions, atrial and ventricular septal defect closures, and new interventions for both electrophysiology (EP) and heart failure.


 September 08, 2014
September 08, 2014