Sony Electronics’ Medical Systems Division showcased a full line of medical grade monitors for radiology applications, including the LMD-DM50, LMD-DM30C and LMD-DM30 models, at the 2014 Society for Imaging Informatics in Medicine (SIIM) meeting, in Long Beach, Calif.
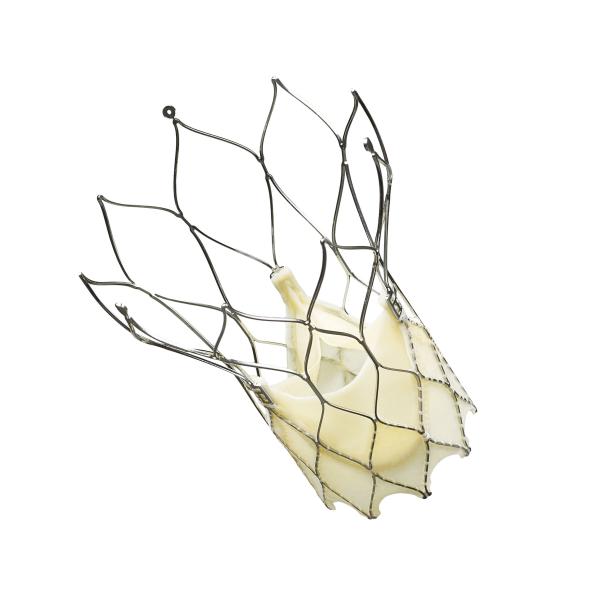
St. Jude Medical Inc. announced that the first patient implants occurred in the Portico Re-sheathable Transcatheter Aortic Valve System U.S. IDE Trial (PORTICO trial).
May 20, 2014 — Siemens Healthcare announced the U.S. Food and Drug Administration (FDA) has cleared the 16- and 32-slice iterations of its Somatom Perspective computed tomography (CT) system.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

May 20, 2014 — GE Healthcare and Tesla Engineering Ltd. are collaborating to produce 7T human whole-body magnetic resonance imaging (MRI) scanners. The companies made the announcement at the joint meeting of the International Society for Magnetic Resonance in Medicine (ISMRM) and the European Society for Magnetic Resonance in Medicine and Biology (ESMRMB) in Milan.
Transcatheter Technologies GmbH, an emerging medical device company that is developing a third-generation transcatheter aortic valve implantation (TAVI) system — Trinity) — announced that its founder and CEO, Wolfgang Goetz, M.D., Ph.D., will be meeting with potential investors, including potential corporate partners, at the EuroPCR annual scientific meeting in Paris, May 20-23.
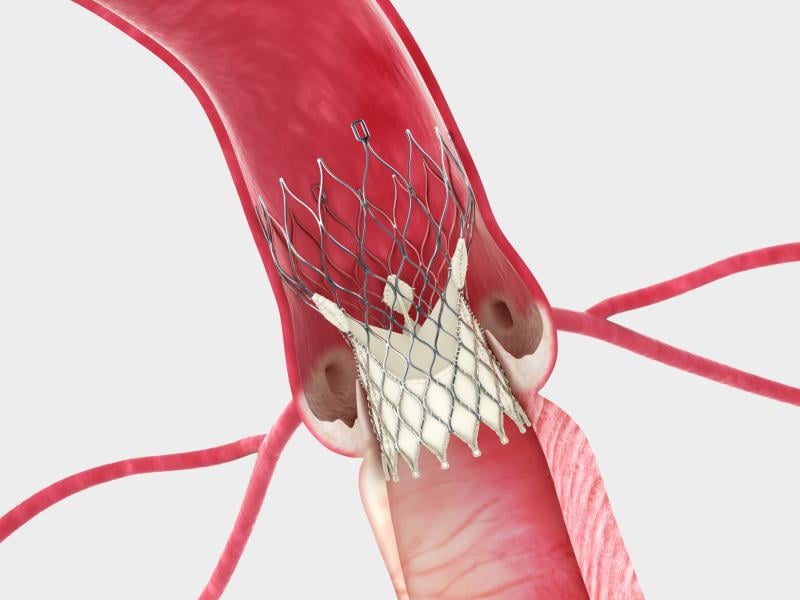
In a surprise move, Edwards Lifesciences Corp. and Medtronic reached an agreement this week to settle all outstanding patent litigation between the companies, including cases related to transcatheter heart valves. The agreement will result in the dismissal of all pending cases or appeals in courts and patent offices worldwide, and includes a provision that the parties will not litigate patent disputes with each other in the field of transcatheter valves for the eight-year duration of the agreement.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

May 19, 2014 — A new study found that adding an electrocardiogram (ECG) to existing pre-participation screening of high school athletes increases the likelihood of identifying disorders associated with sudden cardiac death. The findings were presented at Heart Rhythm 2014, the Heart Rhythm Society’s (HRS) 35th annual scientific sessions.

May 19, 2014 — St. Jude Medical announced that data presented during a late-breaking clinical trial session at the 2014 annual scientific sessions of the Heart Rhythm Society (HRS) found an association between adherence to remote monitoring with the Merlin remote monitoring system and a reduction in patient mortality.
Six independent studies published within the past 10 years have shown a link between whole body vibration and lowered blood pressure.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
For the first time, a minimally invasive transcatheter valve — tested by Baylor Research Institute in Dallas (BRI) — has been shown to save more lives than open-heart surgery, according to new research revealed at the American College of Cardiology’s (ACC) 2014 Scientific Sessions and published in The New England Journal of Medicine.

Fetal heart experts working with the American Heart Assn. have developed guidelines to help healthcare providers care for unborn babies with heart problems, as well as their families.
Advanced Circulatory, makers of medical devices providing Intrathoracic Pressure Regulation (IPR) Therapy, will continue to work with the U.S. Food and Drug Administration (FDA) on a pathway to premarket approval (PMA) for the company’s ResQCPR System, based on recommendations from the FDA Circulatory System Devices Advisory Panel meeting on May 6.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 16, 2014 — New data presented as a late-breaking clinical trial at the Heart Rhythm Society's (HRS) 2014 annual scientific sessions show that an advanced pacing feature exclusive to Medtronic pacemakers significantly delays the progression of persistent atrial fibrillation (AF) in patients with bradycardia. Results from the MINERVA (MINimizERight Ventricular pacing to prevent Atrial fibrillation and heart failure) study found that the Reactive ATP algorithm reduced the development of persistent AF by a 58 percent relative reduction compared to standard pacemakers (p<0.001).
May 16, 2014 — In a move to expand significantly its portfolio of solutions for peripheral interventions, Boston Scientific Corp. has entered into a definitive agreement to acquire the interventional division of Bayer AG for $415 million in cash, including fees for transitional services. The company expects to close the transaction in the second half of 2014, subject to customary closing conditions.
May 15, 2014 — Medweb announced the introduction of several new features for Medweb Collage that will further enable the sharing of information across healthcare systems and promote secure access to patient information from mobile devices. The EHR/EMR Gateway seamlessly integrates patient imaging and reports from Medweb Collage into any EMR (electronic medical record) via industry standards.

 May 20, 2014
May 20, 2014