There is now a 3-D elastic membrane made of a soft, flexible, silicon material that is precisely shaped to match the heart's epicardium. Current technology is two-dimensional and cannot cover the full surface of the epicardium or maintain reliable contact for continual use without sutures or adhesives. The 3-D device was created by an international team of biomedical engineers and materials scientists and Igor Efimov, Ph.D., at the School of Engineering and Applied Science at Washington University in St. Louis.
February 28, 2014 — Mercator MedSystems released pilot study data from a single-arm, open label study of 20 patients suffering with peripheral artery disease (PAD), which reveal sustained, positive clinical outcomes from baseline to 12 months and a one-year primary patency rate of 83 percent.

February 28, 2014 — Igor Efimov, Ph.D., from the School of Engineering & Applied Science at Washington University in St. Louis, and an international team of biomedical engineers and materials scientists have created a 3-D elastic membrane made of a soft, flexible, silicon material that is precisely shaped to match the heart's epicardium, or the outer layer of the wall of the heart.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
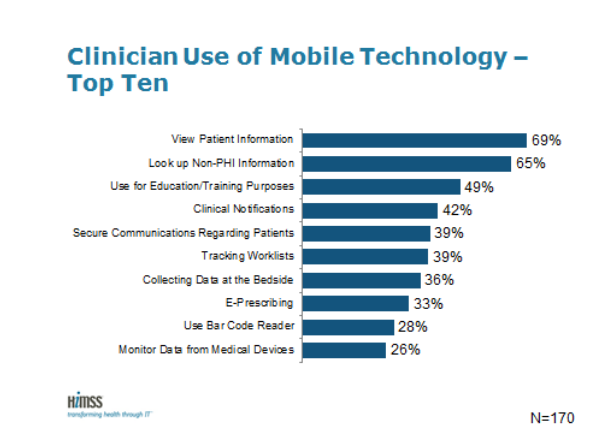
HIMSS Analytics published the results of the third Annual HIMSS Analytics Mobile Survey at its annual conference in Orland, Fla. The survey examines use of mobile devices in provider patient care improvement initiatives. For the first time this year, the survey questions were modified to closely align with the six areas of the mHIMSS Roadmap, a strategic framework for providers to implement mobile and wireless technologies.
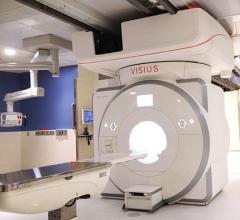
The U.S. Food and Drug Administration (FDA) cleared IMRIS Inc.’s upgraded Visius Surgical Theatre, which integrates Siemens' high-field MR scanners. The core imaging technology based on Siemens Aera 1.5T(tesla) and Skyra 3.0T technology helps IMRIS deliver better image quality with higher signal-to-noise ratio. It also provides faster 3-D image acquisition and improved ease-of-use and workflow during neurosurgical procedures using intraoperative MRI (iMRI).
At HIMSS 2014, Penrad released software that allows the creation of 3-D images of the carotid artery lumen from slides created by conventional 2-D ultrasound.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Archelon Enclosures, a manufacturer of iPad and tablet enclosures for the retail, hospitality and healthcare industries, announced an iPad Air solution to the healthcare industry at HIMSS14.
Approximately one in two patients with atrial fibrillation (Afib) do not optimally reduce their risk of stroke or bleeding when treated with the most widely prescribed oral-anticoagulant therapy, according to a study published online in Circulation.
A new imaging technique for measuring blood flow in the heart and vessels can diagnose bicuspid aortic valve, and may lead to better prediction of complications. A Northwestern Medicine team reported the finding in the journal Circulation. In the study, the authors demonstrated for the first time a previously unknown relationship between heart valve abnormalities, blood flow changes in the heart and aortic disease. They showed that blood flow changes were driven by specific types of abnormal aortic valves, and they were able to directly associate blood flow patterns with aortic diseases.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Despite recent challenges and leadership changes, Merge, according to providers, is performing stronger than ever before. This is according to the newly released KLAS report, Weathering Change: Merge Healthcare Imaging Suite 2014.
Apama Medical is a privately held medical device company formed by Shifamed's medical incubator. It received Chinese notice of allowance for claims covering their low profile electrode assembly that enables the attachment of flexible electrodes to an ablation balloon for energy delivery and monitoring. The Chinese patent will complement an existing patent (U.S. Patent No. 8,295,902) giving Apama broad claim coverage for electrode-based ablation balloons in the United States and China for the treatment of atrial fibrillation (AF). Utilizing this patented electrode technology enables Apama to design an ablation catheter that performs a full pulmonary vein (PV) ablation and rotor ablation in a single-shot.
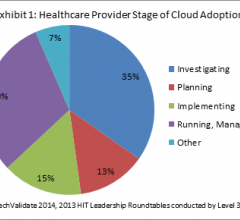
Level 3 Communications Inc. released results of a research study conducted among Level 3 healthcare customers and healthcare IT leaders. The study, which polled respondents about their cloud readiness, revealed that more than half were still investigating cloud options, and that a large majority referenced security concerns as the main barrier to adoption.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
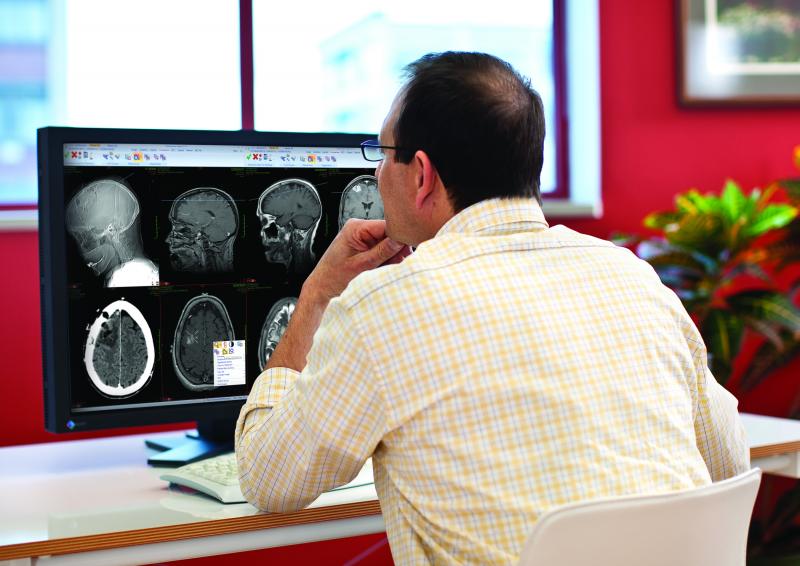
The 25th Annual 2014 HIMSS Leadership Survey examines topics crucial to healthcare leaders including IT priorities, issues driving and challenging technology adoption and IT security. One finding from this year’s survey concerns the perceived impact financial resources are having on IT implementations. A majority of the survey participants (65 percent) reported IT budget increases, which is likely a contributing factor to the transition to a paperless environment. However, a lack of adequate financial resources tops the list of barriers to successful IT implementation. This is a shift from the past two years when the primary IT challenge was insufficient and untrained staffing resources.
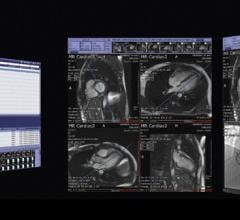
TeraRecon, a provider of medical image management solutions, debuted it’s iNteract+ solution at the 2014 Healthcare Information and Management Systems Society (HIMSS) annual conference.
ScImage announced availability of a Master Patient Index (MPI) Translator that provides healthcare professionals diagnostic imaging data residing in disparate systems, often located at unrelated facilities.

 February 28, 2014
February 28, 2014