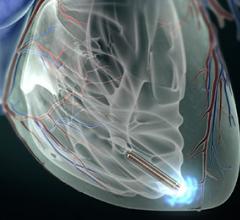
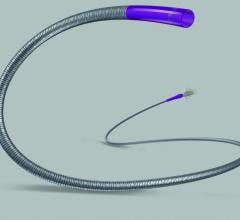
Claret Medical Inc. received CE marking for the Sentinel Cerebral Protection System (CPS) for embolic protection during transcatheter aortic valve replacement (TAVR).
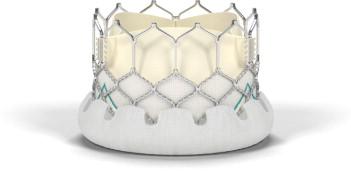
Edwards Lifesciences Corp. received CE marking for its Edwards Sapien 3 transcatheter aortic valve.
Experts presented six-month LEVANT 2 trial results for C. R. Bard Inc.’s Lutonix drug coated balloon (DCB) and provided updates on the ongoing Lutonix Below the Knee (BTK) Clinical Trial at the Leipzig Interventional Course (LINC) in Leipzig, Germany.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Prof. Richard Schilling implanted St. Jude Medical Inc.’s Nanostim retrievable leadless pacemaker at St. Bartholomew’s Hospital, London.
Infraredx Inc.’s TVC Imaging System will be manufactured and distributed in Japan by Nipro Corp. Infraredx intends to commercialize the TVC Imaging System in Japan, China, Korea and other Asia Pacific markets.
BioLeonhardt, a Leonhardt Ventures company based in Los Angeles, unveiled its implantable stem cell pump at the 9th Annual Cell Therapy for Cardiovascular Disease meeting at Columbia University, Jan. 23, 2014.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
An Effectiveness and Cost of ICD Follow-Up Schedule with Telecardiology (ECOST) study indicated the Biotronik Home Monitoring may reduce follow-up costs in patients with implantable defibrillators (ICDs).
The Society for Healthcare Epidemiology of America (SHEA), an infection control organization, issued voluntary recommendations regarding physician attire in Infection Control and Hospital Epidemiology. White coats, neckties, and wrist watches can become contaminated and may potentially serve as vehicles to carry germs from one patient to another.
CorMatrix Cardiovascular Inc. received a patent for an augmented injectable extracellular matrix (ECM) bioscaffold.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The ViScope MD by HD Medical Inc. features murmur detection capability and offers an integrated visual display to allow medical professionals to perform dynamic auscultation and “see” the heart sounds they hear in real-time visual waveforms.
Boston Scientific has received U.S. Food and Drug Administration (FDA) clearance and CE mark approval for its Direxion Torqueable Microcatheter.
Cour Pharmaceutical Development Co. Inc. published data in Science Translational Medicine showing the potential of Immune Modifying Nanoparticles (IMP) to reduce inflammation and promote tissue repair and regeneration in patients who have suffered a heart attack.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The U.S. Food and Drug Administration (FDA) cleared Radius Medical LLC’s Prodigy Support Catheter. Engineered to provide back-up support to guidewires, the Prodigy Support Catheter is intended for use in treating chronic total occlusions.
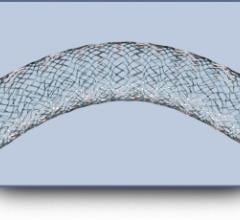
Patients in the European Union (EU) received the first Veniti Vici Venous Stent System implants to treat symptomatic venous outflow obstruction of the lower extremities.
Men with enlarged prostates can find relief with a non-surgical treatment that shrinks the gland, suggests research done on more than 100 patients and presented at the 26th annual International Symposium on Endovascular Therapy (ISET).

 January 28, 2014
January 28, 2014