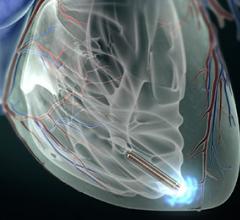
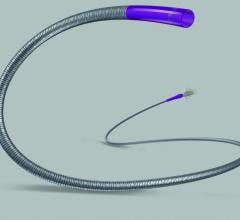
The U.S. Food and Drug Administration (FDA) approved Pressure Products Inc.’s SafeSept Needle Free Transseptal Guidewire for use with any introducer system when crossing the interatrial septum. The device assists in transseptal procedures and is designed to create the primary puncture in the interatrial septum providing access from the right to left side of the heart without using a transseptal needle.
AtheroMed, a developer of catheter technologies for treating peripheral artery disease (PAD), received clearance from the U.S. Food and Drug Administration (FDA) to market the Phoenix Atherectomy System, which allows physicians to treat PAD with a low profile atherectomy catheter that continuously removes diseased material as it is debulked and does not require specialized capital equipment.
Experts from the American Society of Echocardiography (ASE), in collaboration with the Society for Cardiovascular Magnetic Resonance (SCMR) and the Society for Pediatric Radiology (SPR), released a guideline, Multimodality Imaging Guidelines for Patients With Repaired Tetralogy of Fallot: A Report from the American Society of Echocardiography Writing Group, to help optimize lifetime management of patients with repaired tetralogy of Fallot (TOF).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A paper in World Heart Federation’s journal Global Heart advocates for the inclusion of ultrasound in medical education programs to fully realize its benefits as early as possible. The review is by J. Christian Fox, professor of clinical emergency medicine and director of instructional ultrasound, University of California Irvine School of Medicine and colleagues.
Claret Medical Inc. received CE marking for the Sentinel Cerebral Protection System (CPS) for embolic protection during transcatheter aortic valve replacement (TAVR).
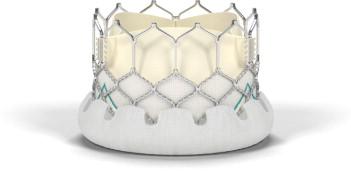
Edwards Lifesciences Corp. received CE marking for its Edwards Sapien 3 transcatheter aortic valve.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Experts presented six-month LEVANT 2 trial results for C. R. Bard Inc.’s Lutonix drug coated balloon (DCB) and provided updates on the ongoing Lutonix Below the Knee (BTK) Clinical Trial at the Leipzig Interventional Course (LINC) in Leipzig, Germany.
Prof. Richard Schilling implanted St. Jude Medical Inc.’s Nanostim retrievable leadless pacemaker at St. Bartholomew’s Hospital, London.
Infraredx Inc.’s TVC Imaging System will be manufactured and distributed in Japan by Nipro Corp. Infraredx intends to commercialize the TVC Imaging System in Japan, China, Korea and other Asia Pacific markets.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
BioLeonhardt, a Leonhardt Ventures company based in Los Angeles, unveiled its implantable stem cell pump at the 9th Annual Cell Therapy for Cardiovascular Disease meeting at Columbia University, Jan. 23, 2014.
An Effectiveness and Cost of ICD Follow-Up Schedule with Telecardiology (ECOST) study indicated the Biotronik Home Monitoring may reduce follow-up costs in patients with implantable defibrillators (ICDs).
The Society for Healthcare Epidemiology of America (SHEA), an infection control organization, issued voluntary recommendations regarding physician attire in Infection Control and Hospital Epidemiology. White coats, neckties, and wrist watches can become contaminated and may potentially serve as vehicles to carry germs from one patient to another.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
CorMatrix Cardiovascular Inc. received a patent for an augmented injectable extracellular matrix (ECM) bioscaffold.
The ViScope MD by HD Medical Inc. features murmur detection capability and offers an integrated visual display to allow medical professionals to perform dynamic auscultation and “see” the heart sounds they hear in real-time visual waveforms.
Boston Scientific has received U.S. Food and Drug Administration (FDA) clearance and CE mark approval for its Direxion Torqueable Microcatheter.


 January 28, 2014
January 28, 2014