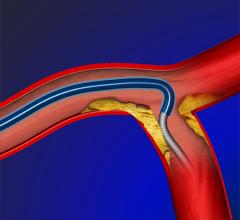
August 14, 2012 — The U.S. Food and Drug Administration (FDA) has granted 510(k) market clearance to Stryker Neurovascular’s Trevo Pro Retriever, indicated for the removal of blood clots. It uses Stentriever technology to optimized clot integration and retrieval in patients experiencing acute ischemic stroke.
August 14, 2012 — Positron Corp. announced the transfer, consolidation and integration of its radiopharmaceutical operations from the Crown Point, Ind. facility to its Lubbock, Texas site.
Biosense Webster Inc. launched the new Carto 3 MEM (Multi-Electrode Mapping) Version in the United States. It is the latest advancement on the Carto 3 system platform.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
August 14, 2012 — The U.S. Food and Drug Administration (FDA) is hosting a public workshop that examines use of absorbable materials in implantable devices for endovascular therapies such as fully absorbable cardiovascular stents, where the stent platform degrades, as well as absorbable coatings.
August 13, 2012 — Analog Devices Inc. (ADI) has introduced a low-power, single-lead, heart rate monitor analog front end (AFE) for a wide range of vital sign monitoring applications. The AD8232 AFE is 50 percent smaller and uses up to 20 percent less power than other solutions.

August 13, 2012 — Providers are increasingly focused on using interventional labs in a hybrid operating room (OR) environment, but better support and training are needed to ensure success in this evolution of technology, suggests some of the findings in a recent KLAS study, “Interventional X-ray 2012: A Continuing Evolution.”
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
August 13, 2012 — To improve magnetic resonance (MR) exam efficiency and image quality, Toshiba America Medical Systems Inc. has received U.S. Food and Drug Administration (FDA) clearance for its high-density 16-element flexible coil system, developed in partnership with NeoCoil. The new coil system makes it easier for clinicians to complete high-quality exams and improve diagnostic efficiency.
August 9, 2012 — CardioDx Inc. announced that Palmetto GBA, a national contractor that administers Medicare benefits, has established coverage for the company’s Corus CAD gene expression test for the evaluation of patients presenting with typical and atypical symptoms suggestive of coronary artery disease (CAD).
August 9, 2012 — The U.S. Food and Drug Administration (FDA) is informing healthcare providers and patients that the indications for use and labeling for the Stryker Wingspan Stent System have changed to limit the use of Wingspan to a narrow, select group of patients and conditions.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
August 9, 2012 — The Harvard Clinical Research Institute (HCRI) announced today the successful completion of randomization in the DAPT study, with the total number of patients randomized exceeding the upper goal set for the study.
Aug. 8, 2012 — The U.S. Food and Drug Administration (FDA) approved the Abbott’s Omnilink Elite Vascular Balloon-Expandable Stent System for the treatment of iliac artery disease. This form of peripheral artery disease (PAD) that affects the lower extremities can progress to where patients experience chronic pain and reduced ability to walk, potentially leading to permanent disability.
Aug. 8, 2012 — Vascular Solutions Inc. launched the SuperCross FT, a new flexible-tip version of its line of SuperCross microcatheters. It is designed to address the majority of complex interventional procedures in which a flexible tipped microcatheter is needed.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Aug. 8, 2012 — The Berlin Heart Group said the U.S. Food and Drug Administration (FDA) has granted approval for its post approval study, a condition of the humanitarian device exemption (HDE) approval that Berlin Heart received for the Excor Pediatric Ventricular Assist Device (VAD) in December 2011.
Aug. 8, 2012 — iHeart Centers acquired the Heart IT WebPAX system for facilitating the management of medical images. The zero-footprint medical image workstation provides Web-based medical image management technology and services to healthcare systems, large hospitals and private clinics.
August 3, 2012 — In a bid to help control healthcare costs, on Oct 1, 2012, the Centers for Medicare and Medicaid Services (CMS) will stop paying hospitals for treating potentially avoidable surgical site infections following cardiac implantable electronic device (CIED) procedures. These include pacemaker and defibrillator implants.


 August 14, 2012
August 14, 2012