April 5, 2010 - If used early, advanced imaging techniques such as computed tomography (CT) scans and magnetic resonance imaging (MRI) might shorten the length of a person's hospital stay and decrease costs associated with hospitalization, according to a study in the April issue of the Journal of the American College of Radiology.
April 2, 2010 – Integrating a sleep disorder diagnostic tool into hospital cardiac care, the MARS Virtual Sleep Lab (VSL) was recently introduced at ACC 2010. It is the first device to provide a streamlined view of quantitative cardiac and sleep apnea analysis from any GE-monitored inpatient bed, helping enhance speed of diagnosis.
April 2, 2010 – The FDA, Society of Nuclear Medicine (SNM) and the Radiological Society of North America (RSNA) are hosting a joint two–topic workshop, April 13–14, at the Natcher Conference Center of the National Institutes of Health, Bethesda, Md.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
April 2, 2010 – A federal jury yesterday found Medtronic’s CoreValve transcatheter aortic valve willfully infringes on patents owned by Edwards Lifesciences. Edwards said it intends to seek a permanent injunction. The jury also awarded Edwards $74 million in damages, and the willfulness finding allows Edwards to seek increased damages of up to three times that amount, the company said.
April 1, 2010 – A new partnership will deliver multimodality cardiovascular image analysis as a part of a forthcoming Web-based service to simplify workflow and save time in cardiology practices.
March 31, 2010 - The number of magnetic resonance imaging (MRI) scans performed increases each year, as does the number of people with implanted cardiac devices.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 31, 2010 — A Web-based suite of clinical and reporting tools for cardiology, cardiovascular and vascular imaging has been deployed at Warren Hospital in Phillipsburg, N.J.
March 31, 2010 — Boston Scientific Corp. recently announced that it has stopped shipment and is retrieving field inventory of all its implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds), after a planned process review revealed that two manufacturing process changes were not submitted for FDA approval.
March 31. 2010 – The FDA granted Clinical Laboratory Improvement Amendments (CLIA)-waived status to expand the use of a point-of-care anti-coagulation monitor to a broader range of clinical settings. The CoaguChek XS Plus system offers connectivity and data management tools to help healthcare professionals manage PT/INR testing.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 30, 2010 – To accelerate development, incubating and commercializing new cardiovascular technology, the Global Cardiovascular Innovation Center (GCIC) plans to open a new building in May. It will house start-up companies developing new solutions for the diagnosis and treatment of cardiovascular disease.
March 30, 2010 – A new coronary stent recently launched in Europe is designed to gain easier access to distal lesions and help restore the artery's natural form. Cordis Corp. launched the Presillion Plus next-generation bare metal stent system. An upgraded delivery system increases the speed of procedures with atraumatic delivery, increased pushability and smoother withdrawal.
March 30, 2010 – The FDA granted an investigational device exemption (IDE) for a clinical study of the Freedom portable driver system to power SynCardia's Total Artificial Heart. The study is designed to demonstrate that stable Total Artificial Heart patients in the U.S. can manage their portable driver outside the hospital environment, including at home and in step-down facilities.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
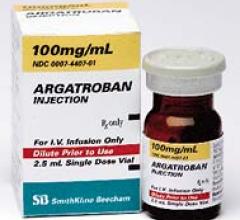
March 30, 2010 – The FDA granted tentative approval for Teva Pharmaceutical’s abbreviated new drug application (ANDA) to market a generic version of the anti-coagulant, Argatroban injection, 100mg/mL.
March 29, 2010 - Optical coherence tomography (OCT) not only has the potential to identify vulnerable plaques, but in countries like Japan, it is already a standard follow up to stenting procedures to determine if the stent has effectively widened the affected artery and the area is free of blood clots.
March 29, 2010 – A lawsuit was settled between Medtronic and AGA Medical Corp. for the alleged infringement of patents concerning the use of stress to restrain self-expanding nitinol medical devices. Under the terms of the settlement AGA will pay an undisclosed amount to Medtronic and Medtronic will grant a nonexclusive license to AGA to use the patent.

 April 05, 2010
April 05, 2010