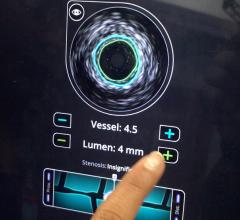
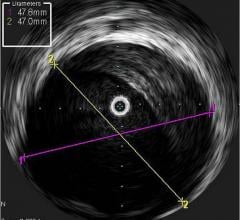
As part of its efforts to expand integration of intravascular ultrasound (IVUS) image guidance with cath lab treatment devices, Volcano Corp. recently announced a supply agreement with ev3, a Covidien company. Volcano will supply its IVUS technology for use in ev3’s TurboHawk plaque excision (atherectomy) systems.
Volcano developed an IVUS-guided balloon catheter, the Vibe RX, which is available in Europe. The company plans to partner with other vendors to create a line of IVUS-guided therapy solutions.
Integrated FFR/OCT Unveiled
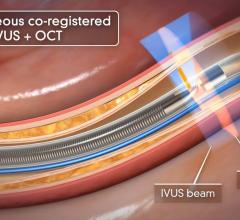
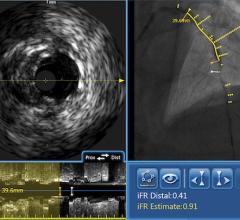
The world’s first system to integrate the functional and anatomical modalities of fractional flow reserve (FFR) and optical coherence tomography (OCT) into one platform was highlighted by St. Jude Medical in May at the Paris Course on Revascularization (EuroPCR) 2011. The Ilumien system enables OCT imaging with the third-generation DragonFly OCT catheter, and FFR measurement through wireless connections, which simplify set-up and measurement through the elimination of connecting cables. The combined technology offers percutaneous coronary intervention (PCI) optimization that helps physicians determine if therapeutic intervention is necessary. The Ilumien is pending European CE mark approval.
FDA Advisory Panel to Review Sapien
A U.S. Food and Drug Administration (FDA) advisory panel is scheduled to review Edwards Lifesciences’ premarket approval (PMA) application for the Sapien transcatheter heart valve on July 20. Edwards submitted a PMA application in the fall of 2010 based on data from the inoperable cohort (Cohort B) of the PARTNER Trial, for approval of this therapy in the treatment of inoperable patients with severe aortic stenosis.
If cleared for commercial use, it would become the first transcatheter aortic valve replacement device in the United States. Transcatheter valve replacement is expected to eventually replace the current standard of open-heart valve replacement surgery.
So far, only one transcatheter valve has been cleared by the FDA – the Medtronic Melody pulmonary valve, which was cleared under a humanitarian device exemption in early 2010.
Medtronic also entered U.S trials for its CoreValve transcatheter aortic valve in late 2010.
FDA Worried About Japanese Devices
The U.S. Food and Drug Administration (FDA) is warning U.S. medical device vendors to beware of possible safety concerns and radioactive contamination of medical devices and components coming from Japan. The FDA said Japan is a critical participant in the global medical device market, but this industry was heavily impacted by the massive March 11, 2011 earthquake and tsunami. The FDA’s Center for Devices and Radiological Health (CDRH) and Center for Biologics Evaluation and Research (CBER) is raising concerns about possible effects these events may have on medical devices, electronic products and device components intended for the U.S. market
Neither CDRH nor CBER has been notified of any defective product or serious adverse events associated with the use of a device related to this disaster. However, given the extent of the devastation, both have concerns about manufacturing conditions as a result of the disaster that could impact the safety and effectiveness of Japanese medical devices. These concerns include radioactive contamination, use of contaminated water, compromise in sterile products during or after production, compromised reliability of product performance (e.g., electrical connectors, microprocessors, alarms, sensors), and damaged and/or disrupted medical device manufacturing facilities.
To read more, visit www.dicardiology.net/article/fda-worries-about-safety-japanese-medical-devices-following-earthquake-tsunami
2011 Best in KLAS Awards
KLAS announced the release of their highly anticipated “2011 Top 20 Best in KLAS Awards: Medical Equipment & Infrastructure” report. The awards are based on data from customer surveys of hospital and clinic executives, administrators, physicians, nurses, clinicians, and other directors and managers interacting with healthcare equipment and infrastructure solutions. Winners applicable to cardiac imaging included:
• Computed Tomography (CT) - 64-slice+: Toshiba Aquilion One
• Magnetic Resonance (MR) - 3.0T: Siemens Magnetom Verio
• MR - 1.5T: Toshiba Vantage
• Ultrasound - General Imaging: GE Healthcare Logiq E9
• Ultrasound - Cardiovascular: GE Healthcare Vivid E9 and the Toshiba Aplio


 September 18, 2025
September 18, 2025