March 15, 2019 – Biotronik announced U.S. Food and Drug Administration (FDA) approval of the Acticor and Rivacor high ...
Cardiac Resynchronization Therapy Devices (CRT)
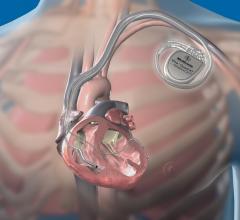
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
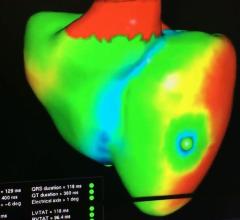
This is a virtual heart with the same electrophysiology characteristics as the real patient being developed to help ...
Interventional cardiac resynchronization therapy (I-CRT) describes the repurposing of a set of tools and techniques ...

To extract or abandon broken or infected implantable, venous electrophysiology (EP) device leads has been a debate for ...
May 22, 2018 — Medtronic plc announced study results showing its AdaptivCRT algorithm is associated with improved ...
May 18, 2018 — Data on the effectiveness of cardiac resynchronization therapy (CRT) in patients with non-left bundle ...
May 18, 2018 — In a new study, cardiac contractility modulation (CCM) therapy was confirmed to significantly improve ...
Here is an aggregation of all the news and late-breaking studies presented at the 2018 Heart Rhythm Society (HRS) Scient ...
April 30, 2018 — LivaNova announced it completed the sale of its cardiac rhythm management (CRM) business to MicroPort ...
April 12, 2018 — Biotronik U.S. and Aziyo announced a strategic agreement allowing Biotronik to distribute Aziyo's ...
February 26, 2018 — The U.S. Food and Drug Administration (FDA) announced that Medtronic is recalling certain implantabl ...
January 3, 2018 — Abbott announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR) ...
November 25, 2017 — Sitting in, or standing close to the charging port of a Tesla electric vehicle did not trigger a ...
October 20, 2017 — Boston Scientific announced new data from the Multisensor Chronic Evaluation in Ambulatory Heart ...
September 27, 2017 — Boston Scientific recently launched the Resonate family of implantable cardioverter defibrillator ...


 March 15, 2019
March 15, 2019