August 29, 2016 — Investment research firm Muddy Waters Capital released a report Aug. 25 saying it believes St. Jude ...
Cardiac Resynchronization Therapy Devices (CRT)
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.

June 28, 2016 — St. Jude Medical Inc. announced CE Mark approval and launch of SyncAV CRT software, designed to build ...
June 23, 2016 — Biotronik announced it was the winner of the Cardiostim Innovation Award in the category “Best Practice ...
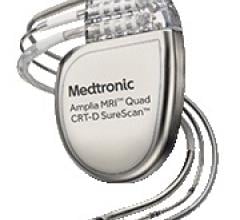
May 11, 2016 — Medtronic plc recently announced it received U.S. Food and Drug Administration (FDA) approval for the ...
May 10, 2016 — The first-ever trial using new SonR hemodynamic sensor technology proves to be safely and effectively ...
May 9, 2016 —St. Jude Medical announced presentation of the MultiPoint Pacing (MPP) Investigational device exemption ...
May 3, 2016 — Biotronik announced U.S. Food and Drug Administration (FDA) approval of Iperia ProMRI HF-T, a cardiac ...
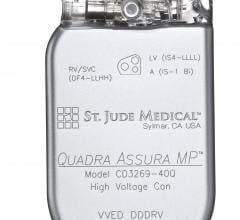
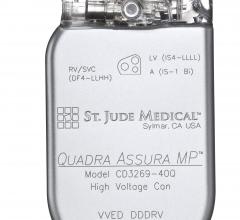
April 18, 2016 — St. Jude Medical Inc. announced the U.S. launch and first U.S. implants of the Quadra Assura MP cardiac ...
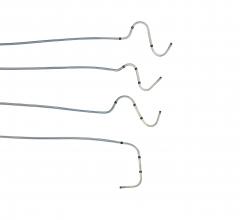
March 29, 2016 — St. Jude Medical Inc. announced CE Mark approval and launch of three new Quartet left ventricular (LV) ...
March 17, 2016 — Medtronic plc announced that it received CE (Conformité Européenne) Mark for the first and only cardiac ...
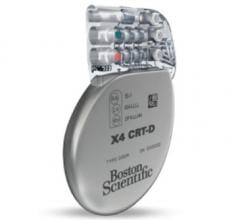
March 4, 2016 — Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for the Acuity X4 ...
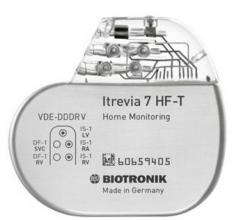
February 25, 2016 — Biotronik announced that its Itrevia 7 HF-T cardiac resynchronization therapy defibrillator (CRT-D) ...
February 3, 2016 — Biotronik announced CE approval for its new Ilivia implantable cardioverter defibrillators (ICDs) and ...
December 14, 2015 — St. Jude Medical Inc. announced CE Mark approval for magnetic resonance (MR) conditional labeling ...
November 4, 2015 — LivaNova PLC, formerly Sorin Group, launched Platinum, a new range of implantable cardiac ...

 August 29, 2016
August 29, 2016