July 13, 2020 - The U.S. Food and Drug Administration (FDA) has approved Abbott's next-generation Gallant implantable ...
Cardiac Resynchronization Therapy Devices (CRT)
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
May 13, 2020 — Cardiac resynchronization therapy (CRT) using biventricular pacing (BVP) or His bundle pacing (HBP) is ...

Cardiovascular disease is the leading cause of death for women in North America, and women with heart failure often ...
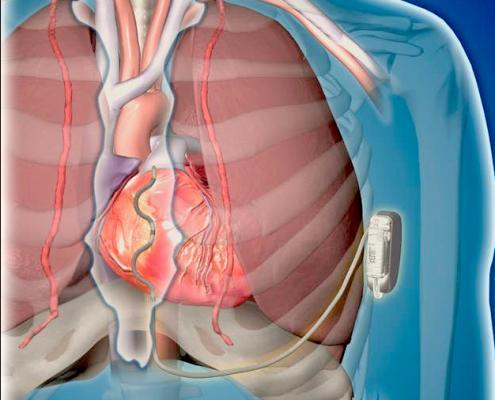
Cardiac rhythm management (CRM) devices in use today are evolving to raise the bar beyond monitoring and managing ...
August 19, 2019 — The U.S. Food and Drug Administration (FDA) granted market clearance the Barostim Neo System for the ...

This is a brief overview of updates on implantable cardioverter defibrillators (ICD), including new technology ...

May 15, 2019 — The Heart Rhythm Society (HRS) had 21 late-breaking study presentations at the 2019 Heart Rhythm ...
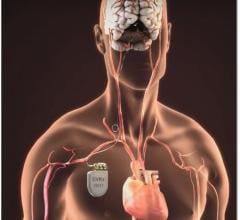
May 15, 2019 — A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The ...
May 15, 2019 - Three new studies show that patients who are medically indicated for implantable heart devices, including ...
May 15, 2019 — A pilot trial has shown His pacing in cardiac resynchronization therapy (CRT) has been shown to ...
May 13, 2019 – Results from a new survey are the first to report a large discrepancy in patient’s knowledge of their ...
May 7, 2019 — The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers ...
April 29, 2019 — Biotronik announced the full commercial launch of the Acticor device family, including Acticor DX and ...
April 18, 2019 – Biotronik announced the European market release of what it calls the world’s smallest implantable ...

March 22, 2019 — The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers ...


 July 13, 2020
July 13, 2020

![A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The new scoring system was presented as a follow up to that study during a late-breaking session at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.](/sites/default/files/styles/content_feed_medium/public/PADIT_Infection_Risk_score.jpg?itok=O1-YAcMm)