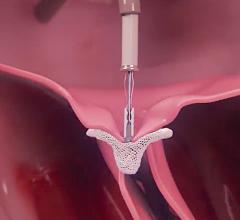
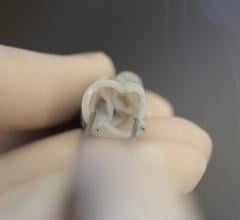
November 9, 2021 — An economic analysis of data from PARTNER 3, a randomized trial comparing transcatheter aortic valve ...
Cardiovascular Surgery
This channel includes news and new technology innovations for cardiac surgery. This includes coronary artery bypass grafts (CABG) and surgical valve replacements.
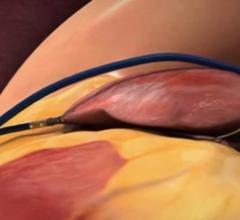
October 27, 2021 — Teleflex Inc. announced the completion of patient enrollment in a clinical study evaluating the ...
October 6, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
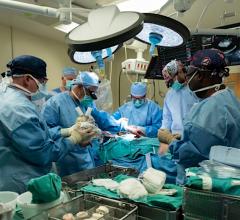
September 22, 2021 — A cardiothoracic surgical team with University of Louisville Health – Jewish Hospital has performed ...
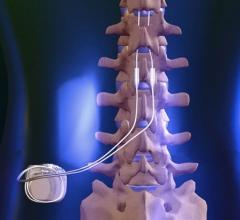
August 4, 2021 — Temporary Spinal cord stimulation (SCS) was effective in suppressing post-operative atrial fibrillation ...

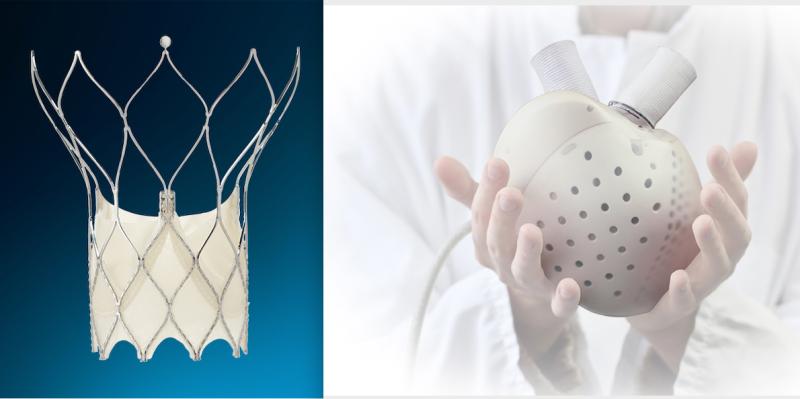
Medtronic announced in June it was stopping the sale and distribution of the Medtronic Heartware HVAD left ventricular ...

Surgical Site Infection (SSI) prevention remains a high priority for cardiac surgeons whose heroic efforts can be ...
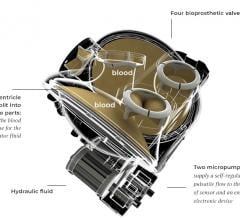
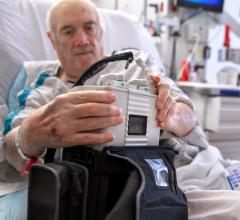
June 18, 2021 – An experimental artificial heart includes an autoregulation control mechanism, or Auto-Mode, that can ...
June 2, 2021 – LivaNova announced June 1 it successfully completed the initial closing of the divestiture of its heart ...
May 15, 2021 — Patients with an elevated risk of stroke due to heart rhythm problems, or atrial fibrillation (AFib) ...
May 4, 2021 – A new study, presented at the 2021 American Association for Thoracic Surgery (AATS) 101st annual meeting ...
April 5, 2021 — Smidt Heart Institute transplant surgeons Dominic Emerson, M.D., and Pedro Catarino, M.D. know how to be ...
Surgeons at Penn State Heart and Vascular Institute were the second group in the nation to implant a newly-designed ...
March 18, 2020 — A groundbreaking new study led by University of Minnesota Twin Cities researchers from both the College ...
March 2, 2021 — Penn State Health Milton S. Hershey Medical Center became the second hospital in the nation to implant a ...


 November 09, 2021
November 09, 2021