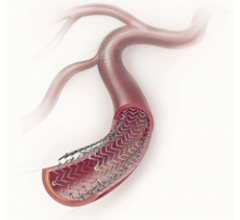
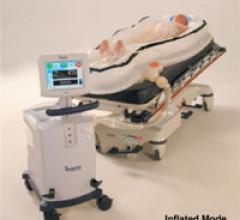
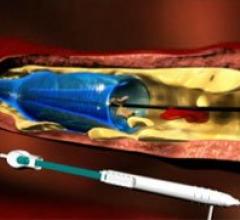
September 16, 2011 — GE Healthcare and Veran Medical Technologies Inc. announced they have formed a strategic alliance ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
September 15, 2011 — Very positive results were reported in the first account of a clinical study evaluating the ...
September 14, 2011 — Miracor Medical Systems GmbH announced today the company has secured $10 million to commercialize ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
September 13, 2011 – An U.S. Food and Drug Administration’s (FDA) will discuss recommendations for Cook Medical Zilver ...
September 13, 2011 – In spite of an increased attention to gender differences in treatment of myocardial infarctions ...
September 13, 2011 — Ziehm Imaging is showcasing the next generation of its mobile C-arm, Ziehm Vision RFD, at the 2011 ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
September 12, 2011 — Life Recovery Systems (LRS) announced it has been awarded a new three-year agreement by Premier ...
September 9, 2011 — Angioslide Ltd. announced the first procedures with its new 5 x 300 mm Proteus device for treating ...
September 8, 2011 – Concentric Medical announced completion of enrollment of the TREVO Study. The TREVO Study ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
September 8, 2011 – Abbott announced the initiation of ABSORB BTK, an international clinical trial evaluating the safety ...
September 8, 2011 – Intra-aortic balloon pump (IABP) counterpulsation prior to percutaneous coronary intervention (PCI) ...
September 8, 2011 – A randomized multicenter, open-label study evaluating the efficacy and safety of prolonged ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
September 8, 2011 – Patients at a high risk for a second stroke who received intensive medical treatment had fewer ...
September 6, 2011 — The European Society of Cardiology (ESC) released the latest version of its guidelines for managing ...
September 6, 2011 — Stryker Corp. has signed a definitive agreement to acquire Concentric Medical for $135 million in an ...

 September 16, 2011
September 16, 2011