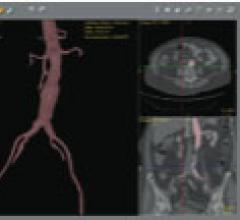
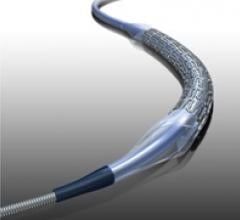
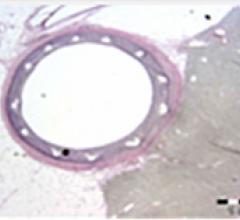
August 17, 2011 – Abbott announced today that the first patient in Japan has been treated with the Absorb bioresorbable ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
August 12, 2011— Simbionix USA Corp., a provider of medical simulation training and education products for medical ...
August 10, 2011 — Hospitals vary markedly when it comes to the rate at which diagnostic coronary angiography or ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
August 10, 2011 – The U.S. Food and Drug Administration (FDA) has approved Abbott’s RX Herculink Elite Renal Stent ...
August 9, 2011 — Boston Scientific Corp. said the U.S. Court of Appeals for the First Circuit has affirmed the dismissal ...
August 5, 2011 — BridgePoint Medical Inc., a Minnesota-based medical device company, has enrolled its first patient in ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
August 5, 2011 — Vessix Vascular Inc. (formerly known as Minnow Medical Inc.), developer of novel percutaneous ...
August 2, 2011 – The U.S. Food and Drug Administration said it recently cleared Abbott Vascular’s RX Herculink Elite ...
Aug. 2, 2011 — New York University (NYU) Langone Medical Center received an $84 million grant from the National Heart ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Paramedics in Bellingham, Wash., used the Lucas device on patient Nancy Olson, who went into cardiac arrest following a ...
July 29, 2011 — InspireMD Inc., developer of the MGuard mesh protective stent system, announced that the first patient ...
July 29, 2011 — Boston Scientific Corporation's board of directors has approved a five-year, $150 million investment to ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 29, 2011 — The U.S. Food and Drug Administration (FDA) this week issued draft guidance that clarifies when changes ...
July 27, 2011 – Micell Technologies Inc. announced that it has completed patient enrollment in its DESSOLVE II CE mark ...
July 27, 2011 – As the number of interventional procedures to diagnose and treat patients increases worldwide, and the ...

 August 17, 2011
August 17, 2011